Cart (0 Items)
Your cart is currently empty.
View ProductsIt looks like you are visiting from outside the EU. Switch to the US version to see local pricing in USD and local shipping.
Switch to US ($)


| Size | 100ug, 1MG |
|---|---|
| Isotype | IgG2-G4-kappa |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | Mammalian cells |
| Applications | Elisa, WB |
| Product name | Eculizumab Biosimilar - Anti-C5 mAb - Research Grade |
|---|---|
| Source | CAS 219685-50-4 |
| Species | Humanized |
| Expression system | Mammalian cells |
| Molecular weight | 148kDa |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Eculizumab,5G1.1,h5G1.1HuG2/G4,C5,anti-C5 |
| Reference | PX-TA1039 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | IgG2-G4-kappa |
| Clonality | Monoclonal Antibody |
Eculizumab Biosimilar – Anti-C5 mAb – Research Grade: A Revolutionary Therapeutic Antibody Targeting Complement Component 5
Eculizumab Biosimilar, also known as Anti-C5 mAb, is a research-grade monoclonal antibody that specifically targets complement component 5 (C5). This biosimilar is a groundbreaking therapeutic antibody that has shown promising results in the treatment of various complement-mediated diseases. In this article, we will delve into the structure, activity, and applications of Eculizumab Biosimilar.
Eculizumab Biosimilar is a recombinant humanized monoclonal antibody that is derived from the parent antibody, Eculizumab. It is composed of two identical heavy chains and two identical light chains, each with a molecular weight of approximately 150 kDa. The antibody has a unique structure with two antigen-binding fragments (Fab) that bind to the C5 protein and a constant fragment (Fc) that mediates effector functions.
Eculizumab Biosimilar exerts its therapeutic effect by specifically targeting and inhibiting the activity of C5, a key component of the complement system. The complement system is a crucial part of the immune system that helps in the defense against pathogens. However, dysregulation of the complement system can lead to various diseases, including paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). Eculizumab Biosimilar blocks the cleavage of C5 into C5a and C5b, preventing the formation of the membrane attack complex (MAC) and the release of pro-inflammatory mediators, thus reducing tissue damage and inflammation.
Eculizumab Biosimilar has shown promising results in the treatment of various complement-mediated diseases, including PNH, aHUS, and myasthenia gravis. PNH is a rare and life-threatening hematological disorder characterized by the destruction of red blood cells, leading to anemia, fatigue, and other complications. Eculizumab Biosimilar has been approved for the treatment of PNH and has shown to improve symptoms and quality of life in patients. aHUS is a rare genetic disorder that causes abnormal blood clots in small blood vessels, leading to damage to vital organs such as the kidneys. Eculizumab Biosimilar has been approved for the treatment of aHUS and has shown to improve kidney function and reduce the risk of organ damage.
Moreover, Eculizumab Biosimilar has shown promising results in the treatment of myasthenia gravis, a neuromuscular disorder characterized by muscle weakness and fatigue. Eculizumab Biosimilar has been shown to improve muscle strength and function in patients with myasthenia gravis.
In conclusion, Eculizumab Biosimilar is a revolutionary therapeutic antibody that specifically targets C5 and has shown promising results in the treatment of various complement-mediated diseases. Its unique structure and mechanism of action make it a promising option for patients who do not respond to conventional treatments. With ongoing research and development, Eculizumab Biosimilar has the potential to improve the lives of patients suffering from complement-mediated diseases.

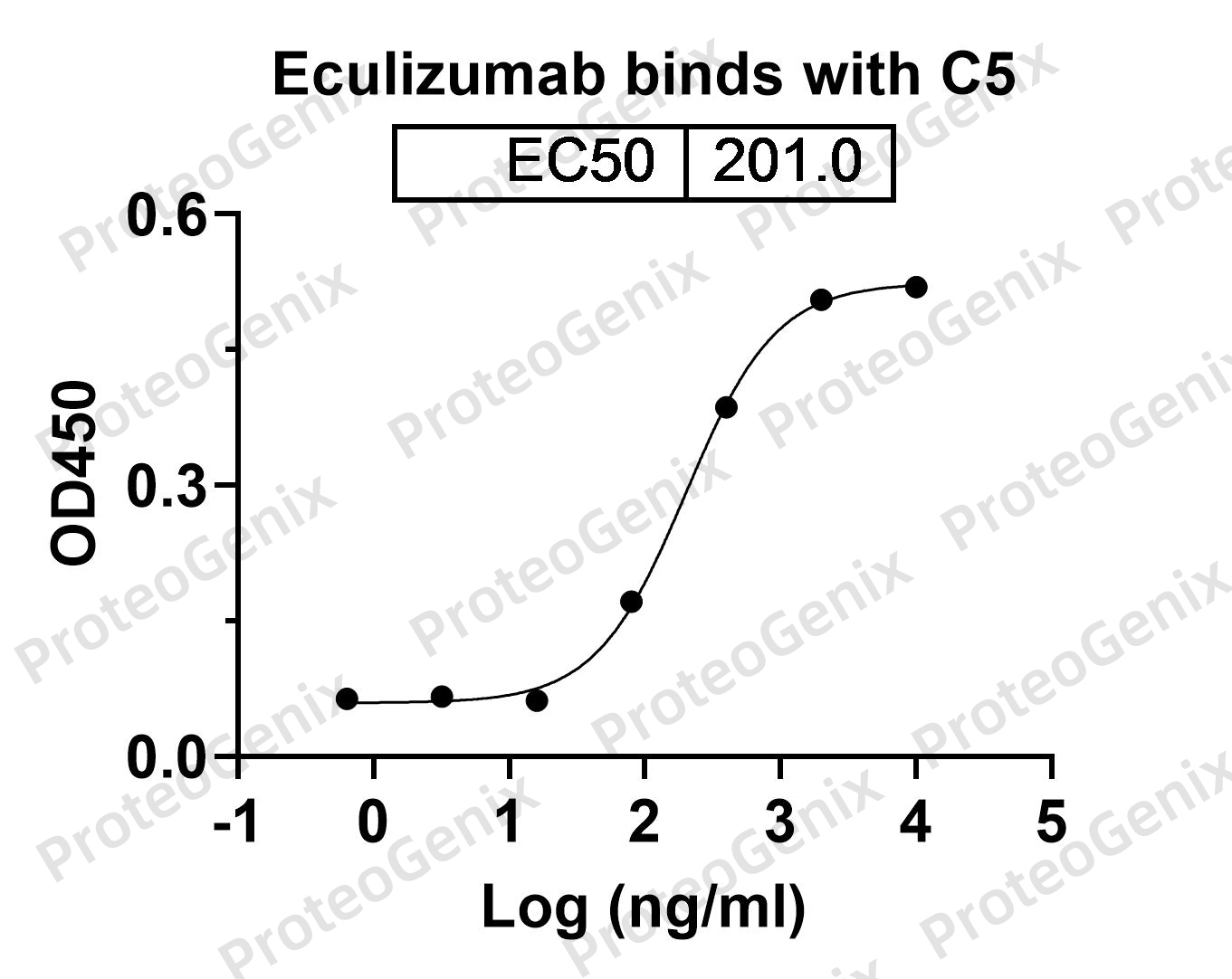
Immobilized Human C5 recombinant protein (cat. No. PX-P5117) at 0.5µg/mL (100µL/well) can bind Eculizumab Biosimilar - Anti-C5 mAb (cat. No. PX-TA1039) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450 giving an EC50 at 201.0M.
Related products
Got a question or need a quote?
Message us and we’ll get back to you 48 hours or less.
Reviews
There are no reviews yet.