Cart (0 Items)
Your cart is currently empty.
View ProductsIt looks like you are visiting from outside the EU. Switch to the US version to see local pricing in USD and local shipping.
Switch to US ($)


| Size | 100ug, 1MG |
|---|---|
| Isotype | IgG4-kappa-[scFv]2 |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | Mammalian cells |
| Applications | Elisa, WB |
| Product name | Tibulizumab Biosimilar - Anti-IL17A, TNFSF13B, CD257 mAb - Research Grade |
|---|---|
| Source | CAS 1849636-24-3 |
| Species | Humanized |
| Expression system | Mammalian cells |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Tibulizumab,LY 3090106,LY-3090106,LY3090106,IL17A, TNFSF13B, CD257,anti-IL17A, TNFSF13B, CD257 |
| Reference | PX-TA1494 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | IgG4-kappa-[scFv]2 |
| Clonality | Monoclonal Antibody |
Introduction to Tibulizumab Biosimilar – Anti-IL17A, TNFSF13B, CD257 mAb – Research Grade Tibulizumab Biosimilar is a monoclonal antibody that specifically targets three key molecules involved in inflammatory and autoimmune diseases: IL17A, TNFSF13B, and CD257. This biosimilar is a highly effective therapeutic agent that has shown promising results in pre-clinical studies and is now being developed for use in clinical trials. In this article, we will explore the structure, activity, and potential applications of Tibulizumab Biosimilar in the field of immunology.
Tibulizumab Biosimilar is a fully human monoclonal antibody, meaning it is derived from human cells and has a high affinity for its target molecules. The antibody is composed of two heavy chains and two light chains, which are connected by disulfide bonds. The variable regions of the antibody are responsible for binding to the specific targets, while the constant regions determine the effector functions of the antibody.
Tibulizumab Biosimilar targets three key molecules involved in inflammatory and autoimmune diseases: IL17A, TNFSF13B, and CD257. IL17A is a pro-inflammatory cytokine that plays a crucial role in the pathogenesis of various autoimmune diseases, including rheumatoid arthritis, psoriasis, and inflammatory bowel disease. TNFSF13B, also known as B-cell activating factor (BAFF), is a cytokine that promotes the survival and maturation of B cells, which are involved in the production of antibodies. CD257, also known as B-cell maturation antigen (BCMA), is a protein expressed on the surface of plasma cells, which are responsible for producing antibodies.
By targeting these three molecules, Tibulizumab Biosimilar inhibits the inflammatory response and suppresses the production of autoantibodies, thereby reducing disease activity and progression. In addition, the antibody can also induce the death of plasma cells, further reducing the production of autoantibodies.
Tibulizumab Biosimilar has shown promising results in pre-clinical studies for the treatment of various autoimmune diseases, including rheumatoid arthritis, psoriasis, and systemic lupus erythematosus. In a mouse model of rheumatoid arthritis, treatment with Tibulizumab Biosimilar significantly reduced joint inflammation and bone destruction. In a mouse model of psoriasis, the antibody inhibited the production of pro-inflammatory cytokines and reduced skin inflammation. In a mouse model of systemic lupus erythematosus, Tibulizumab Biosimilar decreased disease activity and improved survival.
In addition, Tibulizumab Biosimilar has the potential to be used in combination with other therapeutic agents for the treatment of autoimmune diseases. For example, it has been shown to enhance the efficacy of methotrexate, a commonly used drug for rheumatoid arthritis, when used in combination.
In summary, Tibulizumab Biosimilar is a highly effective monoclonal antibody that targets three key molecules involved in inflammatory and autoimmune diseases. Its unique mechanism of action, which includes inhibiting inflammatory cytokines and inducing plasma cell death, makes it a promising therapeutic agent for the treatment of various autoimmune diseases. With ongoing clinical trials, Tibulizumab Biosimilar has the potential to provide a new treatment option for patients suffering from these debilitating conditions.

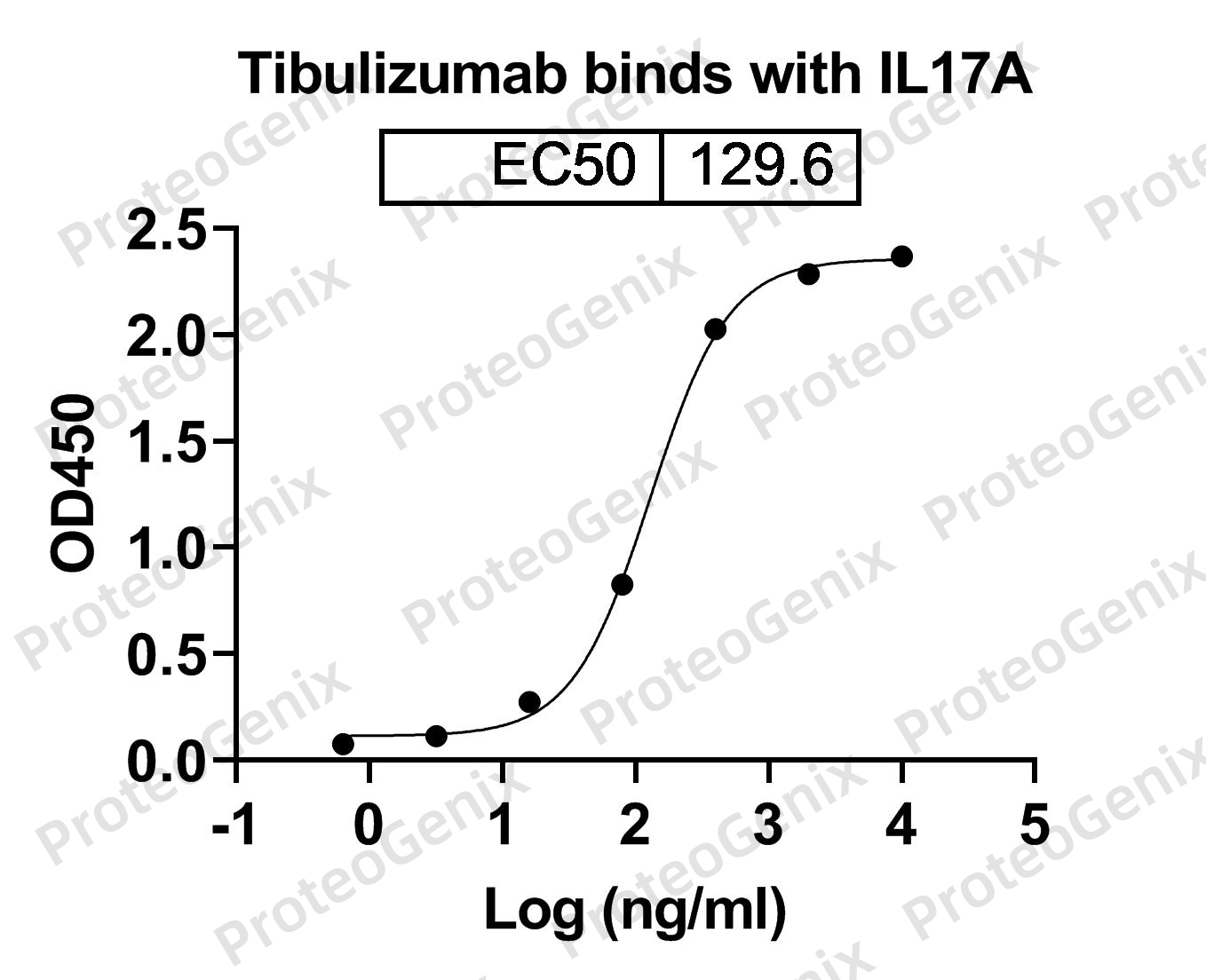
Immobilized IL17A, C-His, recombinant protein (cat. No.PX-P5780) at 0.5µg/mL (100µL/well) can bind to Tibulizumab Biosimilar - Anti-IL17A, TNFSF13B, CD257 mAb (cat. No.PX-TA1494) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450
Related products
Got a question or need a quote?
Message us and we’ll get back to you 48 hours or less.
Reviews
There are no reviews yet.