Cart (0 Items)
Your cart is currently empty.
View ProductsIt looks like you are visiting from outside the EU. Switch to the US version to see local pricing in USD and local shipping.
Switch to US ($)


| Size | 100ug, 1MG |
|---|---|
| Isotype | Bispecific scFv, IgG4;Kappa;Kappa |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | XtenCHO |
| Applications | Elisa, WB |
| Product name | Tebotelimab Biosimilar - Anti-PDCD1;LAG3 mAb - Research Grade |
|---|---|
| Source | CAS 2245725-04-4 |
| Species | Humanized |
| Expression system | XtenCHO |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3 week if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Tebotelimab,MABFRAG HUMAN (FC)MABFRAG HUMANIZED (FAB) ANTI Q15116 (PDCD1_HUMAN)MABFRAG HUMANIZED (FAB) ANTI P18627 (LAG3_HUMAN) (MGD013),,PDCD1;LAG3,anti-PDCD1;LAG3 |
| Reference | PX-TA1722 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | Bispecific scFv ,IgG4;Kappa;Kappa |
| Clonality | Monoclonal Antibody |
Tebotelimab Biosimilar, also known as Anti-PDCD1,LAG3 mAb, is a novel monoclonal antibody that has been developed as a biosimilar to the therapeutic antibody, Tebotelimab. This biosimilar is designed to target the immune checkpoint proteins, Programmed Cell Death Protein 1 (PDCD1) and Lymphocyte Activation Gene 3 (LAG3), which play a crucial role in regulating the immune response. In this article, we will provide a detailed scientific description of Tebotelimab Biosimilar, including its structure, activity, and potential applications.
Tebotelimab Biosimilar is a fully humanized immunoglobulin G4 (IgG4) monoclonal antibody, with a molecular weight of approximately 150 kDa. It is composed of two identical heavy chains and two identical light chains, connected by disulfide bonds. The heavy chain consists of four constant domains (CH1, CH2, CH3, and CH4) and one variable domain (VH), while the light chain contains one constant domain (CL) and one variable domain (VL). The variable domains of both the heavy and light chains are responsible for binding to the target proteins, PDCD1 and LAG3.
Tebotelimab Biosimilar exerts its activity by binding to PDCD1 and LAG3, which are expressed on the surface of T cells. PDCD1, also known as PD-1, is a co-inhibitory receptor that is upregulated on T cells in response to chronic antigen exposure. It interacts with its ligands, PD-L1 and PD-L2, to suppress T cell activation and prevent autoimmunity. LAG3, on the other hand, is a co-inhibitory receptor that is expressed on activated T cells and regulatory T cells (Tregs). It binds to its ligand, MHC class II, and inhibits T cell proliferation and cytokine production.
By targeting PDCD1 and LAG3, Tebotelimab Biosimilar blocks their interaction with their respective ligands, thereby releasing the inhibition on T cell activation. This leads to the activation of T cells and enhances their ability to recognize and kill cancer cells. Additionally, Tebotelimab Biosimilar may also promote the expansion and activation of Tregs, which can suppress the immune response and prevent autoimmune reactions.
Tebotelimab Biosimilar has shown promising results in preclinical studies and is currently being evaluated in clinical trials for the treatment of various types of cancer. Its potential applications include:
1.
Tebotelimab Biosimilar is being developed as a potential immunotherapy for various types of cancer, including solid tumors and hematologic malignancies. By targeting PDCD1 and LAG3, it can enhance the anti-tumor immune response and potentially improve the efficacy of other cancer treatments, such as chemotherapy and radiation therapy.
2. Autoimmune Diseases The inhibition of PDCD1 and LAG3 by Tebotelimab Biosimilar may also have potential applications in the treatment of autoimmune diseases. By blocking the inhibitory signals, it can promote the activation of T cells and restore the balance of the immune system, thereby reducing the severity of autoimmune reactions.
3. Chronic Infections Tebotelimab Biosimilar may also be effective in treating chronic infections, such as HIV and hepatitis B and C. By enhancing T cell activation, it can improve the immune response against the viral infection and potentially lead to viral clearance.
In conclusion, Tebotelimab Biosimilar is a promising monoclonal antibody that targets the immune checkpoint proteins, PDCD1 and LAG3. Its unique structure and mechanism of action make it a potential candidate for the treatment of

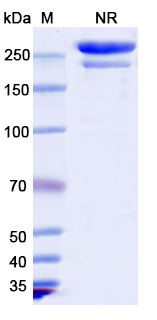
Tebotelimab Biosimilar - Anti-PDCD1;LAG3 mAb, on SDS-PAGE under non-reducing condition. The gel was stained overnight with Coomassie Blue. The purity of the antibody is greater than 95%.
Related products
Got a question or need a quote?
Message us and we’ll get back to you 48 hours or less.
Reviews
There are no reviews yet.