Cart (0 Items)
Your cart is currently empty.
View ProductsIt looks like you are visiting from outside the EU. Switch to the US version to see local pricing in USD and local shipping.
Switch to US ($)
We start your project by assessing feasibility and providing you with a guaranteed yield estimate so you can confidently plan downstream scale-up.

| Step | Content | Timeline | Deliverables |
|---|---|---|---|
| Audit |
|
<1 week |
|
| Optional: In silico developability assessment |
|
~1-2 weeks |
|
| Optional: High-throughput production and characterization |
|
~3-5 weeks |
|
| Gene Synthesis and Transient Expression |
|
~3 week |
|
| Generation of Stable Pools |
|
~8 weeks |
|
| Single Cell Clone Screening by VIPS™ |
|
~8-10 weeks |
|
| RCB Preparation and QC |
|
~1 weeks |
|
| RCB Stability Study |
|
~ 4-5 weeks |
|
| Antibody Analytics |
|
~ 2-3 weeks |
|
We ensure you get the yield and quality you need by giving you full control at every stage of our cell line development workflow. At each checkpoint, you can pause, adjust, or redirect the project to keep it perfectly aligned with your goals.
And if you prefer, you can even test the produced antibodies in your own laboratory, under your experimental conditions!
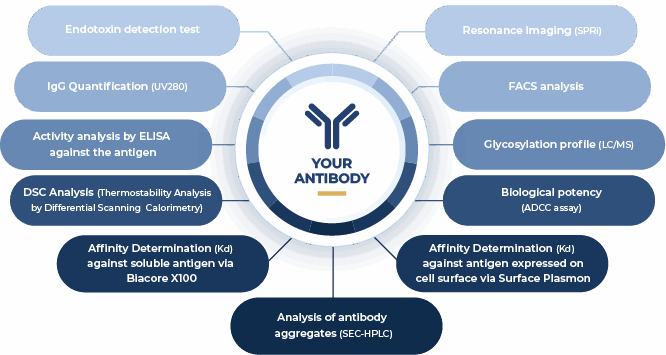
At ProteoGenix, we ensure your antibodies are reliable from the start through developability assessments.
For the final deliverable, we provide a range of quality controls for your antibody, giving you confidence as you move to the next steps of scale-up production.
Looking for the perfect partner to develop your stable cell lines?
Download our exclusive decision-making guide now
and ensure you make the right choice.
Boosting production yields by 1,000-fold
Discover how a biotech company went from 6 mg/L in transient expression to 8.35 g/L while maintaining >90% purity thanks to our stable cell line development service.
Navigating the Complexity of Stable Cell Line Development
Dive into the key steps to successfully develop your stable cell line and discover why VIPS™ is a game-changer for ensuring true monoclonality and regulatory confidence.
The ultimate stable cell line development guide
A practical walkthrough of every critical step, risk points, and strategic solutions to master your stable cell line development.
The 5 pillars of excellence in stable cell line development
A clear, visual summary of the key traits to ensure efficiency, reliability, and safety of your production process.
