Cart (0 Items)
Your cart is currently empty.
View ProductsIt looks like you are visiting from outside the EU. Switch to the US version to see local pricing in USD and local shipping.
Switch to US ($)


| Size | 100ug, 1MG |
|---|---|
| Isotype | IgG1-nd |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | Mammalian cells |
| Applications | Elisa, WB |
| Product name | Teprotumumab Biosimilar - Anti-IGF1R, CD221 mAb - Research Grade |
|---|---|
| Source | CAS 1036734-93-6 |
| Species | Homo sapiens |
| Expression system | Mammalian cells |
| Molecular weight | 148kDa |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Teprotumumab,RO4858696-000,IGF1R, CD221,anti-IGF1R, CD221 |
| Reference | PX-TA1242 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | IgG1-nd |
| Clonality | Monoclonal Antibody |
Teprotumumab Biosimilar: A Novel Anti-IGF1R Antibody for Therapeutic Targeting of CD221 Teprotumumab Biosimilar, also known as Anti-IGF1R, CD221 mAb – Research Grade, is a promising novel antibody that has shown great potential in the field of targeted therapy. This biosimilar antibody is designed to specifically target the insulin-like growth factor 1 receptor (IGF1R) and has been developed as a biosimilar to Teprotumumab, a FDA-approved monoclonal antibody for the treatment of thyroid eye disease. In this article, we will discuss the structure, activity, and potential applications of this innovative biosimilar antibody.
Teprotumumab Biosimilar is a recombinant, fully humanized IgG1 monoclonal antibody with a molecular weight of approximately 150 kDa. It is composed of two identical heavy chains and two identical light chains, connected by disulfide bonds. The antibody is produced in Chinese hamster ovary (CHO) cells using recombinant DNA technology.
The variable region of Teprotumumab Biosimilar is highly specific for the extracellular domain of IGF1R, which is overexpressed in various types of cancer cells. The constant region of the antibody is responsible for mediating effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), which are important for its therapeutic activity.
Teprotumumab Biosimilar has been shown to bind to IGF1R with high affinity and specificity, inhibiting the binding of insulin-like growth factor 1 (IGF1) and insulin-like growth factor 2 (IGF2) to the receptor. This leads to the downregulation of the IGF1R signaling pathway, which is known to play a critical role in cancer cell proliferation, survival, and metastasis.
In addition to its direct anti-tumor effects, Teprotumumab Biosimilar has also been shown to enhance the activity of other anti- cancer therapies, such as chemotherapy and radiation therapy. This is due to its ability to sensitize cancer cells to these treatments by inhibiting the IGF1R-mediated survival pathways.
The primary application of Teprotumumab Biosimilar is in the treatment of various types of cancer, including lung, breast, and prostate cancer. It has also shown promising results in clinical trials for the treatment of thyroid cancer, where IGF1R is known to be overexpressed.
Moreover, Teprotumumab Biosimilar has the potential to be used in combination with other anti- cancer therapies, as it has been shown to enhance their activity and overcome resistance to treatment. This could lead to improved outcomes and prolonged survival for cancer patients.
Aside from its anti-tumor effects, Teprotumumab Biosimilar has also shown promise in the treatment of other diseases, such as diabetes and metabolic disorders. This is due to the role of IGF1R in regulating glucose metabolism and insulin signaling.
Teprotumumab Biosimilar is a novel anti-IGF1R antibody with a unique structure and highly specific activity. Its potential applications in cancer treatment and other diseases make it a promising therapeutic option for patients. Further research and clinical trials are needed to fully explore the potential of this biosimilar antibody and its role in improving patient outcomes.

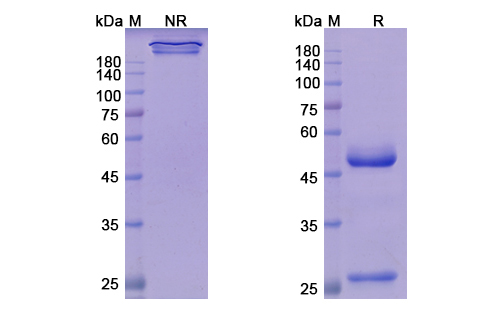
Teprotumumab Biosimilar - Anti-IGF1R, CD221 mAb, on SDS-PAGE under reducing and non-reducing condition. The gel was stained overnight with Coomassie Blue. The purity of the antibody is greater than 95%.

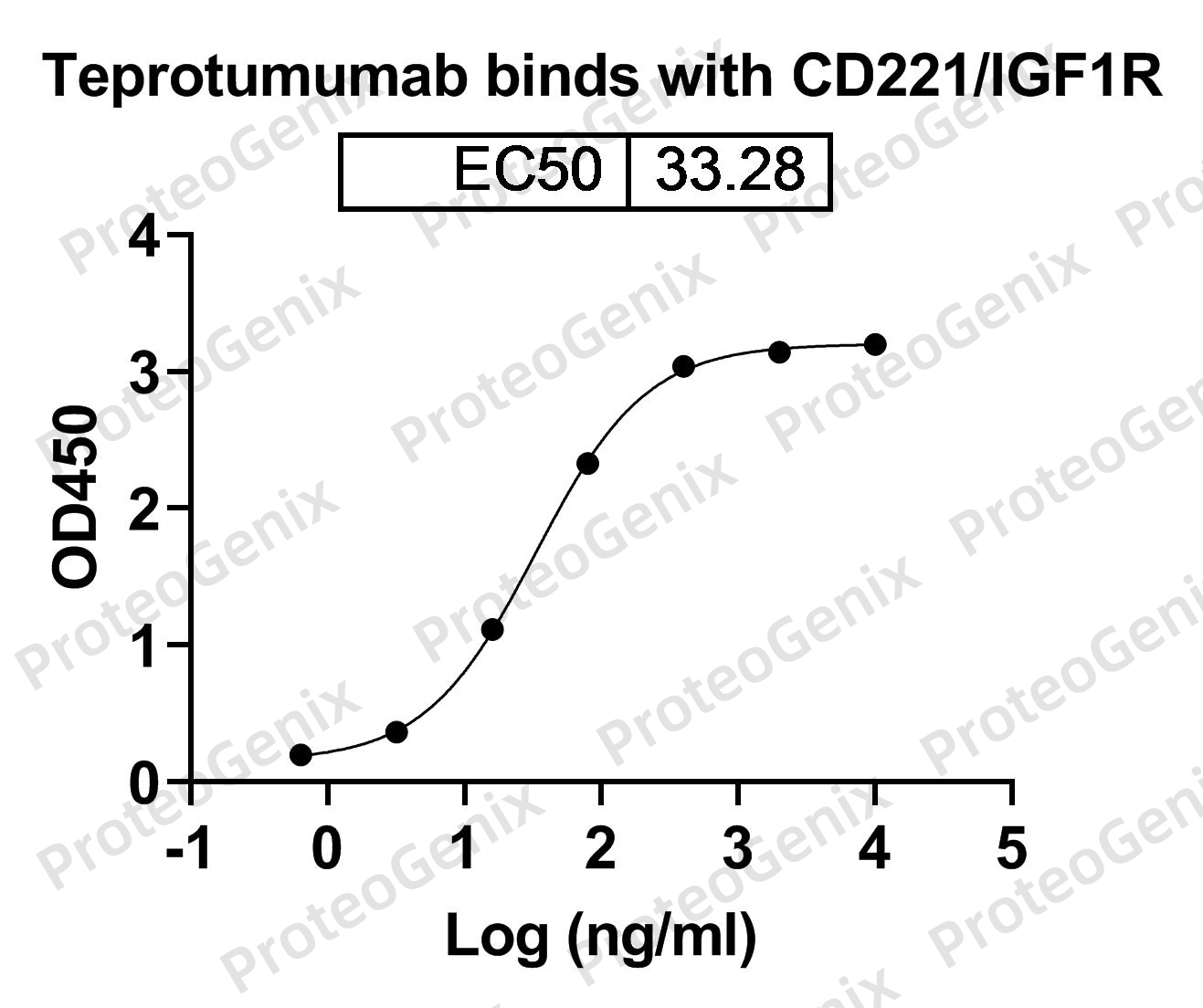
Immobilized IGF1R recombinant protein (cat. No.PX-P5185) at 0.5µg/mL (100µL/well) can bind to Teprotumumab Biosimilar - Anti-IGF1R, CD221 mAb (cat. No.PX-TA1242) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450
Related products
Got a question or need a quote?
Message us and we’ll get back to you 48 hours or less.
Reviews
There are no reviews yet.