Cart (0 Items)
Your cart is currently empty.
View ProductsIt looks like you are visiting from outside the EU. Switch to the US version to see local pricing in USD and local shipping.
Switch to US ($)


| Size | 100ug, 1MG |
|---|---|
| Isotype | IgG2b-nd |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | Mammalian cells |
| Applications | Elisa, WB |
| Product name | Tenatumomab Biosimilar - Anti-TNC, HXB mAb - Research Grade |
|---|---|
| Source | CAS 1412891-40-7 |
| Species | Mus musculus |
| Expression system | Mammalian cells |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Tenatumomab,ST2146,TNC, HXB,anti-TNC, HXB |
| Reference | PX-TA1161 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | IgG2b-nd |
| Clonality | Monoclonal Antibody |
Tenatumomab Biosimilar, also known as Anti-TNC, HXB mAb, is a monoclonal antibody that has been developed as a biosimilar to Tenatumomab, a therapeutic antibody used in the treatment of cancer. This biosimilar has been designed to target the same therapeutic target as Tenatumomab, but with a more cost-effective and accessible option for patients.
Tenatumomab Biosimilar is a recombinant, humanized IgG1 monoclonal antibody that has been engineered to target the Tenascin-C (TNC) protein. It consists of two heavy chains and two light chains, with a molecular weight of approximately 150 kDa. The antibody has a specific binding site for TNC, which is located in the extracellular matrix of tumor cells.
The main activity of Tenatumomab Biosimilar is its ability to bind to TNC, a protein that is overexpressed in various types of cancer, including breast, lung, and colon cancer. TNC plays a crucial role in tumor growth, invasion, and metastasis, making it an attractive therapeutic target. By binding to TNC, Tenatumomab Biosimilar blocks its activity and inhibits tumor growth and metastasis.
Tenatumomab Biosimilar has shown promising results in preclinical studies and is currently being evaluated in clinical trials for the treatment of various types of cancer. It has the potential to be used as a monotherapy or in combination with other cancer treatments, such as chemotherapy or radiation therapy. The biosimilar has also been shown to have a favorable safety profile, making it a promising option for cancer patients.
Several clinical trials have been conducted to evaluate the safety and efficacy of Tenatumomab Biosimilar. In a phase I study, the biosimilar showed promising results in patients with advanced solid tumors, with a manageable safety profile. Another phase I study in patients with advanced breast cancer showed that Tenatumomab Biosimilar had a good safety profile and demonstrated antitumor activity. Currently, a phase II trial is ongoing to evaluate the efficacy and safety of the biosimilar in patients with advanced or metastatic breast cancer.
Tenatumomab Biosimilar has been developed as a biosimilar to Tenatumomab, a therapeutic antibody that has been approved for the treatment of advanced melanoma. Biosimilars are highly similar to their reference product in terms of structure, function, and clinical efficacy, but may have some minor differences. The main advantage of Tenatumomab Biosimilar over Tenatumomab is its lower cost, making it a more accessible option for patients.
Tenatumomab Biosimilar, also known as Anti-TNC, HXB mAb, is a promising biosimilar that has been developed as a cost-effective alternative to Tenatumomab for the treatment of cancer. Its specific targeting of TNC, a protein involved in tumor growth and metastasis, makes it a promising option for cancer therapy. Ongoing clinical trials will provide further insights into the efficacy and safety of this biosimilar, and if successful, it has the potential to improve the treatment options for cancer patients.

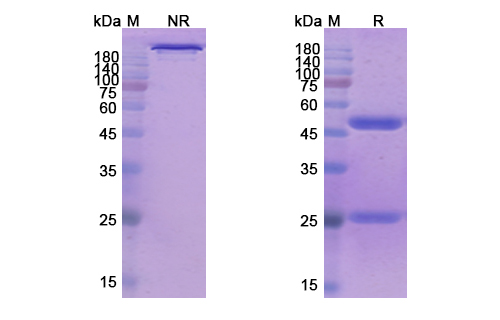
Tenatumomab Biosimilar - Anti-TNC, HXB mAb, on SDS-PAGE under reducing and non-reducing condition. The gel was stained overnight with Coomassie Blue. The purity of the antibody is greater than 95%.
Related products
Got a question or need a quote?
Message us and we’ll get back to you 48 hours or less.
Reviews
There are no reviews yet.