Cart (0 Items)
Your cart is currently empty.
View ProductsIt looks like you are visiting from outside the EU. Switch to the US version to see local pricing in USD and local shipping.
Switch to US ($)


| Size | 100ug, 1MG |
|---|---|
| Brand | ProteoGenix |
| Product type | Recombinant Proteins |
| Expression system | XtenCHO |
| Applications | Elisa, WB |
| Product name | Lenercept Biosimilar - Anti-TNF fusion protein - Research Grade |
|---|---|
| Expression system | XtenCHO |
| Purity | >90% by SDS-PAGE. |
| Buffer | 0.01M PBS, pH 7.4. |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | 4°C for short term; -20°C for long term |
| Brand | ProteoGenix |
| Aliases /Synonyms | anti-TNF, Tumor necrosis factor ligand superfamily member 2, N-terminal fragment, ICD2, NTF, TNF-a, TNF-alpha, Tumor necrosis factor, TNFSF2, TNFA, Cachectin, ICD1 |
| Reference | PX-TA2017 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | Fusion - [TNFRSF1A (tumor necrosis factor receptor (TNFR) superfamilly member 1A, TNFAR, TNF-RI, TNF-R-I, p55, CD120a)]2 - IGHG1 Fc (Fragment constant) |
Lenercept Biosimilar is a research grade anti-TNF fusion protein that has been developed as a potential therapeutic agent for various inflammatory and autoimmune diseases. It is a biosimilar of etanercept, a widely used anti-TNF drug, and is designed to have similar structure and activity.
Lenercept Biosimilar is a recombinant fusion protein consisting of two components – a soluble TNF receptor and the Fc region of human immunoglobulin G1 (IgG1). The TNF receptor component is composed of the extracellular domain of the human TNF receptor 2 (TNFR2) and the transmembrane and cytoplasmic domains of the human TNF receptor 1 (TNFR1). The Fc region, on the other hand, is responsible for extending the half-life of the protein in the body by binding to the neonatal Fc receptor (FcRn).
The structure of Lenercept Biosimilar is similar to that of etanercept, with the only difference being the source of the TNF receptor component. While etanercept is derived from the extracellular domain of the human TNF receptor 2, Lenercept Biosimilar uses the extracellular domain of the human TNF receptor 2. This difference in the source of the TNF receptor component does not affect the overall structure and function of Lenercept Biosimilar.
Lenercept Biosimilar acts as a competitive inhibitor of TNF, a pro-inflammatory cytokine that plays a crucial role in various inflammatory and autoimmune diseases. The TNF receptor component of Lenercept Biosimilar binds to TNF with high affinity, preventing it from binding to its natural receptors on the cell surface. This blocks the downstream signaling pathways activated by TNF, thereby reducing inflammation and tissue damage.
The Fc region of Lenercept Biosimilar also plays a role in its mechanism of action. It extends the half-life of the protein in the body, allowing for prolonged inhibition of TNF and providing sustained therapeutic effects.
Lenercept Biosimilar has shown promising results in preclinical studies and is currently being evaluated for its therapeutic potential in various inflammatory and autoimmune diseases, including rheumatoid arthritis, psoriasis, Crohn’s disease, and psoriatic arthritis.
In a phase I clinical trial, Lenercept Biosimilar was found to be safe and well-tolerated in healthy volunteers. It also showed comparable pharmacokinetic and pharmacodynamic profiles to etanercept, further supporting its potential as a biosimilar of the widely used anti-TNF drug.
Further clinical trials are needed to establish the efficacy and safety of Lenercept Biosimilar in treating specific diseases. If successful, it has the potential to provide a more affordable treatment option for patients, as biosimilars are typically priced lower than their reference biologics.
Lenercept Biosimilar is a promising anti-TNF fusion protein that has been developed as a potential therapeutic agent for various inflammatory and autoimmune diseases. Its structure and mechanism of action are similar to that of etanercept, but with a different source of the TNF receptor component. Further clinical studies will determine its efficacy and safety in treating specific diseases, and if successful, it has the potential to provide a more affordable treatment option for patients.

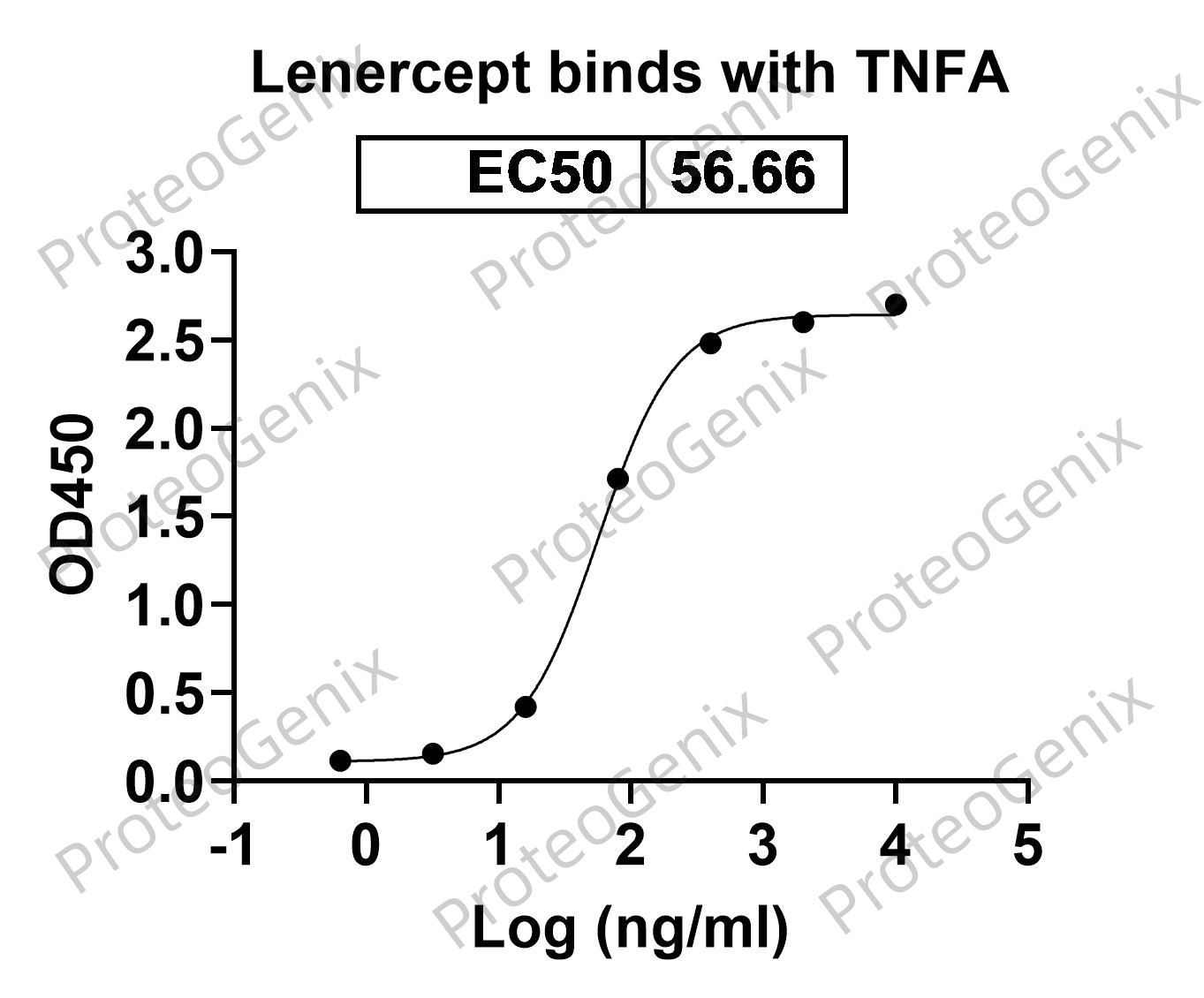
Immobilized TNFa / TNF-alpha, N-His, recombinant protein (cat. No.PX-P5961) at 0.5µg/mL (100µL/well) can bind to Lenercept Biosimilar - Anti-TNF fusion protein (cat. No.PX-TA2017) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450
Related products
Got a question or need a quote?
Message us and we’ll get back to you 48 hours or less.
Reviews
There are no reviews yet.