Cart (0 Items)
Your cart is currently empty.
View ProductsIt looks like you are visiting from outside the EU. Switch to the US version to see local pricing in USD and local shipping.
Switch to US ($)
Client
Mid-size Canadian biotech
Sector
Biotechnology
Research Domain
Oncology
Timeframe
3 weeks
Key Processes
A mid-size Canadian biotech developing a therapeutic antibody for colorectal cancer approached us to accelerate their candidate selection process. They had generated 18 antibody sequences in-house and needed a rapid way to evaluate their production yields. Instead of testing production internally, which would have cost more time and resources, they sent the sequences to us for small-scale expression and yield comparison.
Time pressure to produce and test many antibody variants in parallel
Securing enough material for downstream tests
Need for consistent quality (purity >90%) across all candidates
Ensuring scalability from pilot to larger volumes without loss of performance
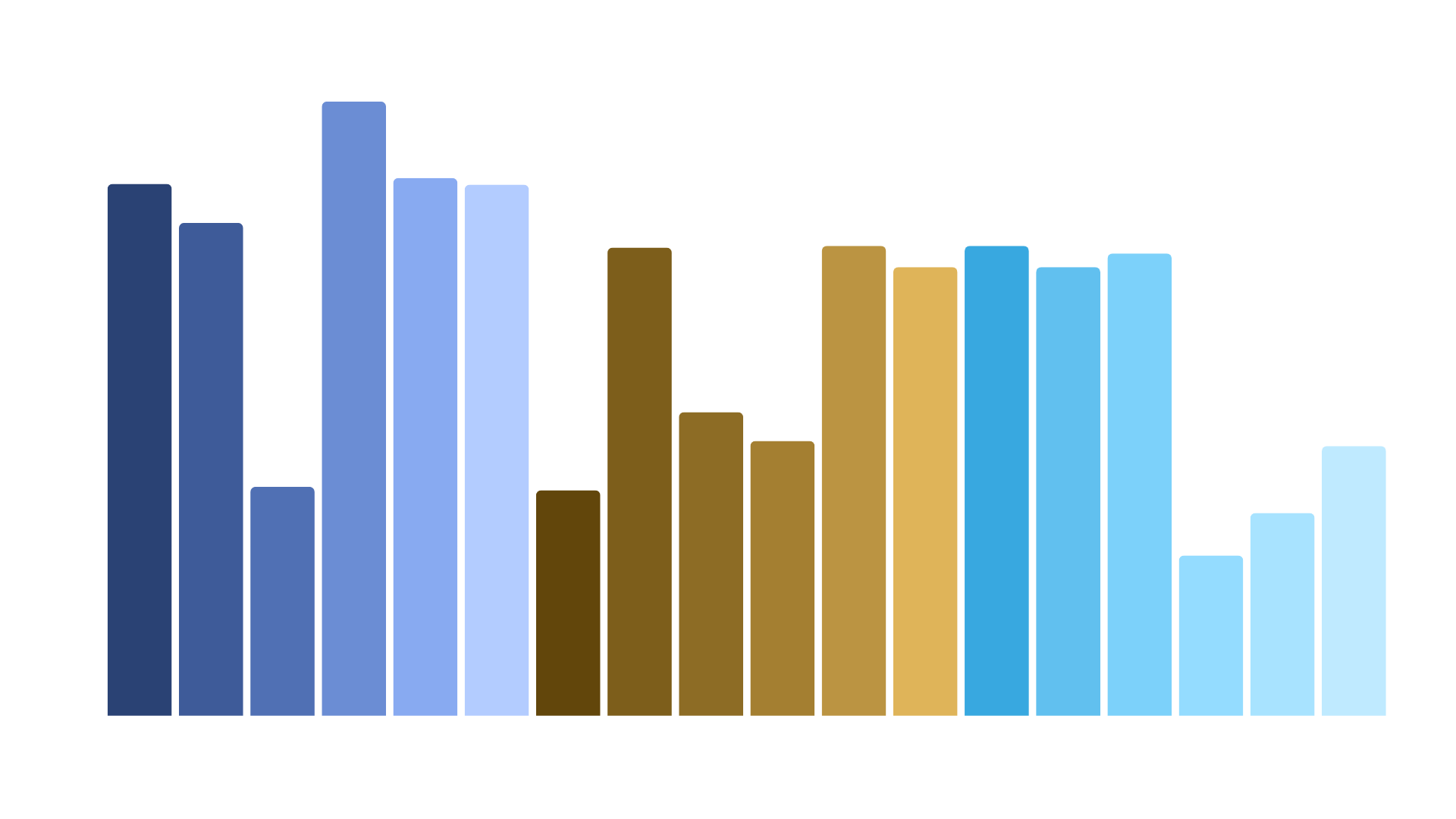
Using the XtenCHO® Race platform, ProteoGenix successfully produced all 18 antibody variants in parallel, achieving yields ranging from approximately 2 mg to over 7 mg per 30 mL culture with purity above 90%. Several variants demonstrated particularly promising expression levels, ensuring the client received sufficient quantities of material to continue their downstream assays. With these results, the biotech was able to confidently narrow their focus and select 8 antibodies for detailed affinity testing, effectively streamlining their development pipeline.
Early yield evaluation with XtenCHO® Race enabled a rapid first selection of candidates. This allowed the client to save time and money while keeping the quality and consistency needed to confidently advance their therapeutic program with their new variants selection.
