Cart (0 Items)
Your cart is currently empty.
View ProductsIt looks like you are visiting from outside the EU. Switch to the US version to see local pricing in USD and local shipping.
Switch to US ($)






| Brand | ProteoGenix |
|---|---|
| Product type | XtenCHO |
| Size | XtenCHO ™ Starter kit |
| Product name | XtenCHO ™ Race Starter kit |
|---|---|
| Expression system | Mammalian |
| Delivery condition | Blue ice (+4°C) |
| Delivery lead time in business days | Europe: 2-4 business days USA & Canada: 2-4 business days Rest of the world: 5-10 business days |
| Storage condition | +4°C and DI |
| Brand | ProteoGenix |
| Host species | Mammalian cells |
| Reference | PX-XTE-R-001 |
| Note | For research use only. |
| Kit Content |
|
| Limited Use Label Licenses | Limited Use Label License for internal Research and Development Use of Mammalian Cell Lines |
The XtenCHO™ Race mammalian expression system has been meticulously developed to provide a swift and economical solution for recombinant protein expression. It is particularly suited for a diverse array of applications, ranging from small- to medium-scale production and the expedited screening of drug candidates and protein variants.
Leveraging over 28 years of expertise, XtenCHO™ Race integrates the high expression capabilities and resilience of a novel Chinese Hamster Ovary (CHO) cell line with the superior efficiency of our proprietary transfection reagent, XtenFect™. This combination yields numerous advantages, including:
• Yield increases of up to 10 times compared to other commercial systems*
• Enhanced plasmid retention (over 14 days of transient gene expression – TGE) achieved through highly effective transfection that maintains cell viability, improves plasmid stability, and elevates protein expression without requiring additional feeds
• Short protocol in 8 days only
• Streamlined protocols with fewer steps, designed to simplify and expedite your workflow, supported by robust and responsive technical assistance
• Elimination of case-by-case optimization due to the high expression levels of the pXen1 vector
• Optimal protein folding and human-like post-translational modifications
• Cost-effective biopharmaceutical-grade production
• Improved safety owing to the enhanced viral resistance of CHO cells in comparison to Human Embryonic Kidney (HEK) cells
• Flexible and scalable production capabilities (ranging from 1 mL to 10 L) tailored to meet your specific requirements
• Worldwide shipping within 24 hours or less
In comparison to other commercial systems, XtenCHO™ has demonstrated superior performance, achieving a yield increase of 1.5 to 9.7-fold*:
• Record productivity for IgG-like antibodies (1.8 g/L for Garetosmab)
• Bispecific antibodies exceeding 100 mg/L
• Humanized antibody variants surpassing 200 mg/L
• Non-antibody proteins yielding over 100 mg/L for ACE2 with excellent enzymatic activity
• Pentameric IgM antibodies
• And more
The XtenCHO™ Race kit features our exclusive chemically defined, animal-free expression media designed to ensure optimal performance. It also includes our effective expression booster, XtenCHO™ Enhancer, along with control plasmids to provide maximum control and flexibility.
For many years, biomolecules entering phase I clinical trials have experienced a low success rate of under 10%. Nevertheless, nearly three-quarters of biopharmaceuticals that have received approval are produced using CHO-based systems. An analysis of the data indicates that CHO-based systems may effectively address early developmental challenges that commonly impede success.
XtenCHO™ Race enables the production of high-quality recombinant proteins for comprehensive early bioanalysis, allowing for the early identification of potential developability issues and enhancing your likelihood of success.
With ultra-high yields, rapid production, and protein folding and P&trade comparable to clinical-grade biopharmaceuticals, XtenCHO™ is exceptionally well-suited for the swift evaluation of drug candidates or protein variants generated through random or targeted mutagenesis.
Although bioassays serve as essential research and monitoring tools across various industries—including medicine, food production, and environmental analysis—they often depend on antibodies produced natively in hybridomas, resulting in lower purity and limited accuracy and precision. However, the landscape is evolving. Through its CHO-based recombinant expression, XtenCHO™ Race enables you to achieve higher yields, enhance batch-to-batch consistency, and improve purity in a cost-effective manner. With intelligent protocols, XtenCHO™ Race will integrate smoothly into your in-house workflows and adapt to your protein production requirements.

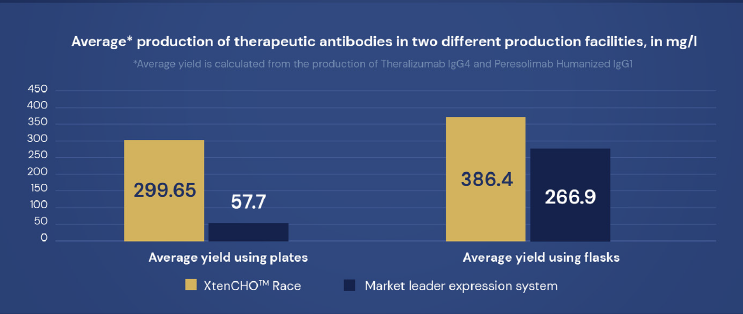
Crude production yields in mg/L obtained in 24-well plates cultures without feeding (Octet Analysis) Yields in flask (better aeration) will be 1.5 to 2.5 Higher for Human/humanized IgG1/IgG4 (depending on the antibody considered)

XtenCHO™ Race ensures consistent and high quality yields, seamlessly adapting to different production scales and formats.
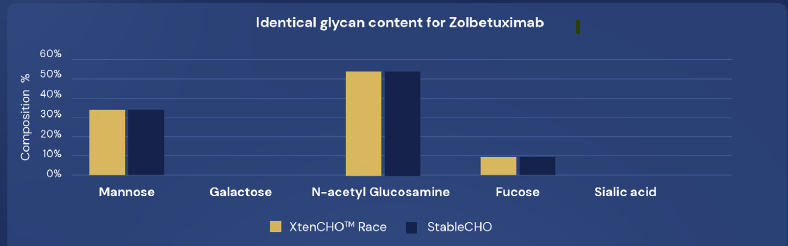
XtenCHO™ Race offers a high level of quality that is maintained for 2 weeks.
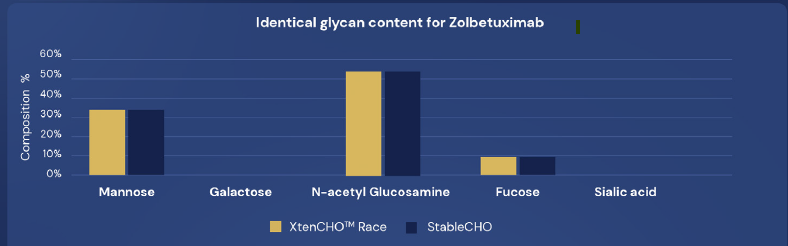
XtenCHO™ Race achieves twice the yields as early as the 8th day of production. NB: Crude production yields obtained in flasks.
Related products
Got a question or need a quote?
Message us and we’ll get back to you 48 hours or less.
Reviews
There are no reviews yet.