Cart (0 Items)
Your cart is currently empty.
View ProductsIt looks like you are visiting from outside the EU. Switch to the US version to see local pricing in USD and local shipping.
Switch to US ($)


| Size | 100ug, 1MG |
|---|---|
| Brand | ProteoGenix |
| Product type | Recombinant Proteins |
| Expression system | XtenCHO |
| Applications | Elisa, WB |
| Product name | Tulinercept Biosimilar - Anti-TNF fusion protein - Research Grade |
|---|---|
| Expression system | XtenCHO |
| Purity | >90% by SDS-PAGE. |
| Buffer | 0.01M PBS, pH 7.4. |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | 4°C for short term; -20°C for long term |
| Brand | ProteoGenix |
| Aliases /Synonyms | anti-TNF, Tumor necrosis factor ligand superfamily member 2, N-terminal fragment, ICD2, NTF, TNF-a, TNF-alpha, Tumor necrosis factor, TNFSF2, TNFA, Cachectin, ICD1 |
| Reference | PX-TA2023 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | Fusion - [TNFRSF1B (tumor necrosis factor receptor (TNFR) superfamily member 1B, TNFBR, p75, CD120b)]2 - IGHG1 Fc (Fragment constant) |
Tulinercept Biosimilar is a novel biologic drug that has gained significant attention in the field of immunology and biotechnology. It is an anti-tumor necrosis factor (TNF) fusion protein that has shown promising results in pre-clinical studies for the treatment of various inflammatory and autoimmune diseases. In this article, we will discuss the structure, activity, and potential applications of Tulinercept Biosimilar in detail.
Tulinercept Biosimilar is a recombinant fusion protein that consists of two components – a soluble TNF receptor (TNFR) and a human IgG1 Fc domain. The TNFR component is derived from the extracellular domain of human TNFR1, which is responsible for binding to TNF. The Fc domain, on the other hand, is responsible for extending the half-life of the drug and enhancing its stability. The two components are linked together by a flexible linker, resulting in a single molecule with a molecular weight of approximately 150 kDa.
The structure of Tulinercept Biosimilar is similar to that of the approved biologic drug, Etanercept, which is used for the treatment of various inflammatory diseases. However, Tulinercept Biosimilar has a higher binding affinity for TNF and a longer half-life, making it a potentially more effective and convenient treatment option.
The primary mechanism of action of Tulinercept Biosimilar is to bind and neutralize TNF, a pro-inflammatory cytokine that plays a crucial role in the pathogenesis of many autoimmune and inflammatory diseases. TNF is known to induce the production of other inflammatory cytokines, activate immune cells, and promote tissue damage, making it a prime therapeutic target for these conditions.
Tulinercept Biosimilar works by binding to TNF and preventing it from interacting with its receptors on immune cells. This leads to a decrease in the production of inflammatory cytokines and a reduction in immune cell activation, ultimately resulting in a decrease in disease symptoms and progression.
Tulinercept Biosimilar has shown promising results in pre-clinical studies for the treatment of various inflammatory and autoimmune diseases, including rheumatoid arthritis, psoriasis, Crohn’s disease, and ulcerative colitis. It has also been studied for its potential in treating other conditions such as ankylosing spondylitis, juvenile idiopathic arthritis, and psoriatic arthritis.
In addition to its potential as a therapeutic drug, Tulinercept Biosimilar also has potential applications in research and diagnostic settings. Its high binding affinity for TNF makes it a valuable tool for studying the role of TNF in various diseases and for developing diagnostic assays to measure TNF levels in patient samples.
Tulinercept Biosimilar is a promising anti-TNF fusion protein with a unique structure and mechanism of action. It has the potential to be a more effective and convenient treatment option for various inflammatory and autoimmune diseases, and its applications in research and diagnostics make it a versatile and valuable drug. Further clinical studies are needed to fully understand its safety and efficacy, but the early results are promising, and Tulinercept Biosimilar has the potential to make a significant impact in the field of immunology and biotechnology.

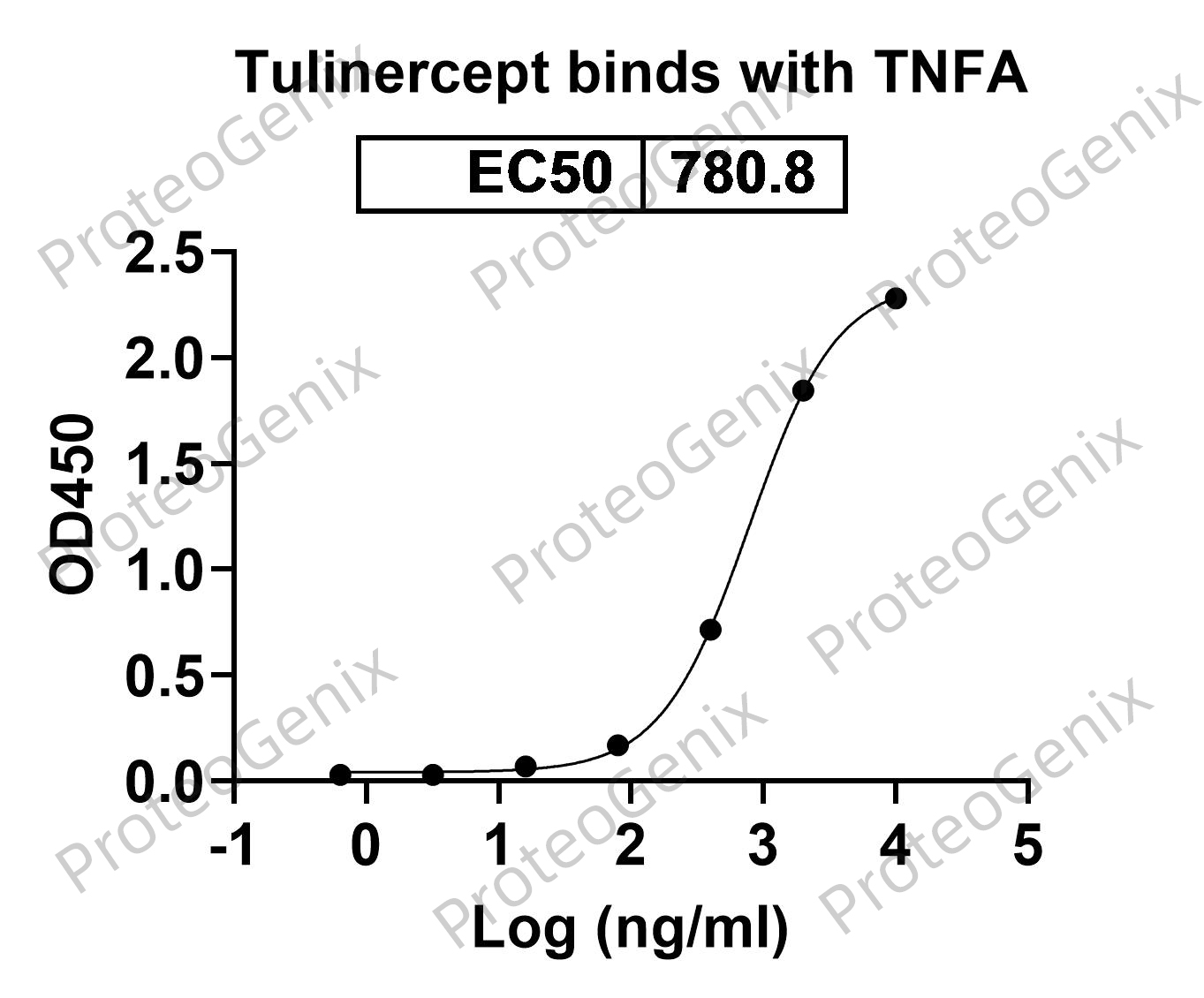
Immobilized TNFa / TNF-alpha, N-His, recombinant protein (cat. No.PX-P5961) at 0.5µg/mL (100µL/well) can bind to Tulinercept Biosimilar - Anti-TNF fusion protein (cat. No.PX-TA2023) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450
Related products
Got a question or need a quote?
Message us and we’ll get back to you 48 hours or less.
Reviews
There are no reviews yet.