 Antibody-drug conjugates
Antibody-drug conjugates
Antibody-enzyme conjugates for therapeutic applications
Antibody-enzyme conjugates represent a small but growing field of study within the vast topic of payloads for antibody-drug conjugate (ADCs) production. These therapies exist in two main forms, the first one leveraging the principle of prodrug/drug systems and the second making use of enzymes with naturally high cytotoxic activities. In this article, we provide an overview of the major advantages and limitations of these systems as well as provide insights regarding current trends in this field.
Main forms of antibody-enzyme conjugate therapy
Antibody-enzyme conjugated therapies exist in two main forms:
- Antibody-directed enzyme prodrug therapy (ADEPT)
- Antibody-directed toxic enzyme fusion protein
The first form of this therapy was proposed as early as 30 years ago. ADEPT was initially devised as an alternative to early antibody-drug conjugates (ADCs). These early combinatorial therapies suffered mostly from low internalization rates and poor cellular trafficking, while ADEPT therapies were devised to forgo the need for internalization and, instead, act directly in the tumor’s extracellular environment by converting prodrugs to potent cytotoxic agents on site. The second form of this therapy was proposed later. In this approach, the enzyme moiety is toxic by itself and the antibody moiety is responsible for directing it towards cancer antigen-bearing cells.
Antibody-directed enzyme prodrug therapy (ADEPT)
The principle of enzymes and prodrug systems is straightforward: a low toxicity drug is administered systemically to the patient and the same drug is later converted to a potent cytotoxic agent by specific enzymes. By coupling the enzyme to antibodies, researchers ensure that this conversion happens solely within tumors to avoid systemic toxicity.
Through this approach, the potent drug is generated in the extracellular space and diffuses quickly through the cellular membranes, leading to the death of cells expressing the target antigen or antigen-negative cells in the vicinity (known as the bystander effect).
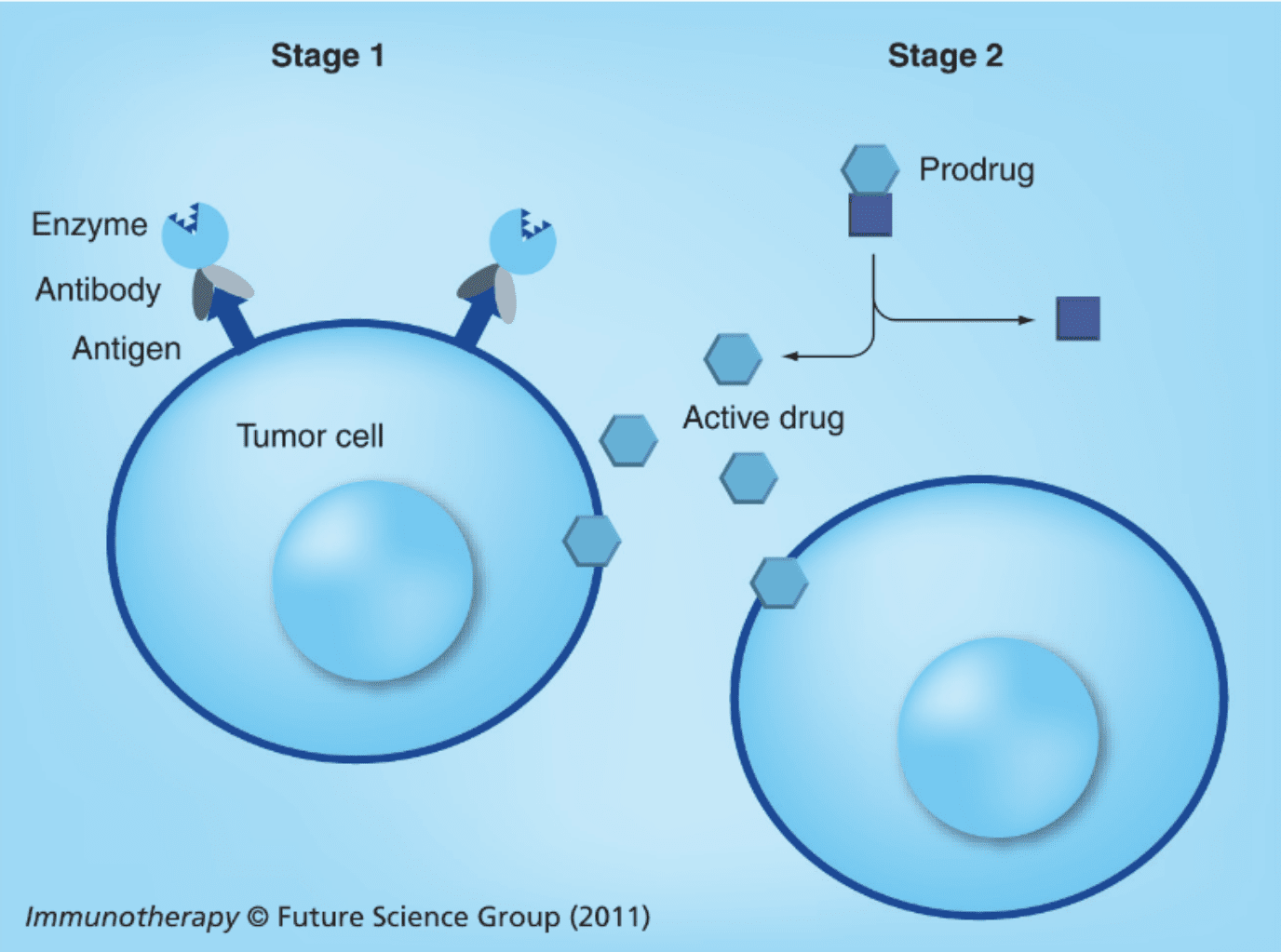
Both mammalian and non-mammalian enzymes have been tested in ADEPT systems. Of mammalian systems, human β-glucuronidase is the most commonly used. Enzymes of non-mammalian origin have also been extensively used such as nitroreductase (with a mammalian homolog), carboxypeptidase G2, and β-lactamase. Of these, only the carboxypeptidase G2 (CPG2) has been applied and studied in clinical settings.
CPG2 is a bacterial enzyme isolated from Pseudomonas sp. responsible for catalyzing the cleavage of folates. Preclinical studies of CPG2 conjugated to anti-hCG (human gonadotrophin) or anti-CEA (carcinoembryonic antigen) have been carried out in combination with a CMDA or BIP prodrug. Studies in mouse models showed complete tumor regression. In clinical trials, these combinatorial therapies appeared to be well tolerated but the results in terms of tumor regression were inconclusive.
As seen with immunotoxins, one of the major drawbacks of ADEPT systems based on bacterial enzymes is their natural immunogenicity. Several patients receiving these therapies showed an increased production of anti-CPG2 antibodies, which is known to limit the therapeutic efficacy of these systems. Moreover, the ADEPT construct must be administered in low amounts to avoid accumulation in the patient’s blood or non-specific sites, because if they do, the conversion of the prodrug may occur in healthy tissues, significantly increasing the risk of systemic toxicity.
For this reason, before ADEPT systems become viable, it is necessary to implement strategies for the deimmunization (removal of immunogenic residues) of the enzyme moiety and the activation of this catalytic part of ADEPT solely after interaction with cancer-associated antigens.
Antibody-directed toxic enzyme fusion proteins
Unlike ADEPT systems, antibody-directed toxic enzyme systems need to be internalized by cancer cells in order to exert their cytotoxic activity. In these systems, the enzyme moiety acts as the cytotoxic agent while the antibody moiety is responsible for directing it towards cancer cells. To date, the major classes of toxic enzymes used for these applications are RNase, barnase, onconase, granzyme B, and caspases, among others.
RNase-based conjugated therapies are the most widely studied within this class. These are RNA-cleaving enzymes that play a key role in the regulation of gene expression in mammalian cells. In their free form, these enzymes are not naturally cytotoxic because they have not developed mechanisms for entering cancer cells. When internalized by cells, RNases have proven to be potent cytotoxins affecting several cellular processes including protein synthesis. However, the challenge lies in facilitating their translocation through the cellular membrane.
The fusion of RNases with antibodies was created with this purpose in mind. The combination of the two biomolecules is known as immunoRNase whereas a tumor-selective antibody is designed to deliver the RNase to the cytoplasm of malignant cells. The exact mechanism through which internalized RNases kill is still poorly understood, but experts agree that it may depend on the cell’s stage in the growth cycle.
In comparison to other antibody-drug conjugates (ADCs) such as immunotoxins or ADEPT systems, immunoRNases are known to be less immunogenic leading to higher therapeutic efficacy. Interestingly, human RNase activity is controlled by the cytosolic RNase inhibitor (RI). For this reason, the use of RI-resistant human or bacterial RNases has shown more promising results in vitro. An example of a bacterial RNase that has found promising results for ADC applications is barnase, an RNase produced by Bacillus amyloliquefaciens and highly resistant to human RI. Despite its potent cytotoxic activity, bacterial RNases such as barnase may cause immunogenic reactions in patients. For this reason, more studies are necessary to understand how to best leverage their activity without scarifying therapeutic efficacy.
Concluding remarks
Within the field of ADC development, considerably little attention has been given to antibody-enzyme conjugates. These complex systems have been tested in vitro and at the preclinical level and less is known about their efficacies in clinical settings. Nevertheless, these conjugates remain a potent alternative to conventional ADCs due to their significantly different mechanism of action which may help alleviate the burden imposed by drug resistance that often leads to treatment failures or relapses in anticancer therapies.
- Andrady, C. et al. Antibody-enzyme fusion proteins for cancer therapy. Immunotherapy. 2011; 3(2): 193-211. doi: 10.2217/imt.10.90
- Sharma, S. K. and Bagshawe, K. D. Antibody Directed Enzyme Prodrug Therapy (ADEPT): Trials and tribulations. Adv Drug Deliv Rev. 2017; 118:2-7. doi: 10.1016/j.addr.2017.09.009