 CAR-T cell therapy
CAR-T cell therapy
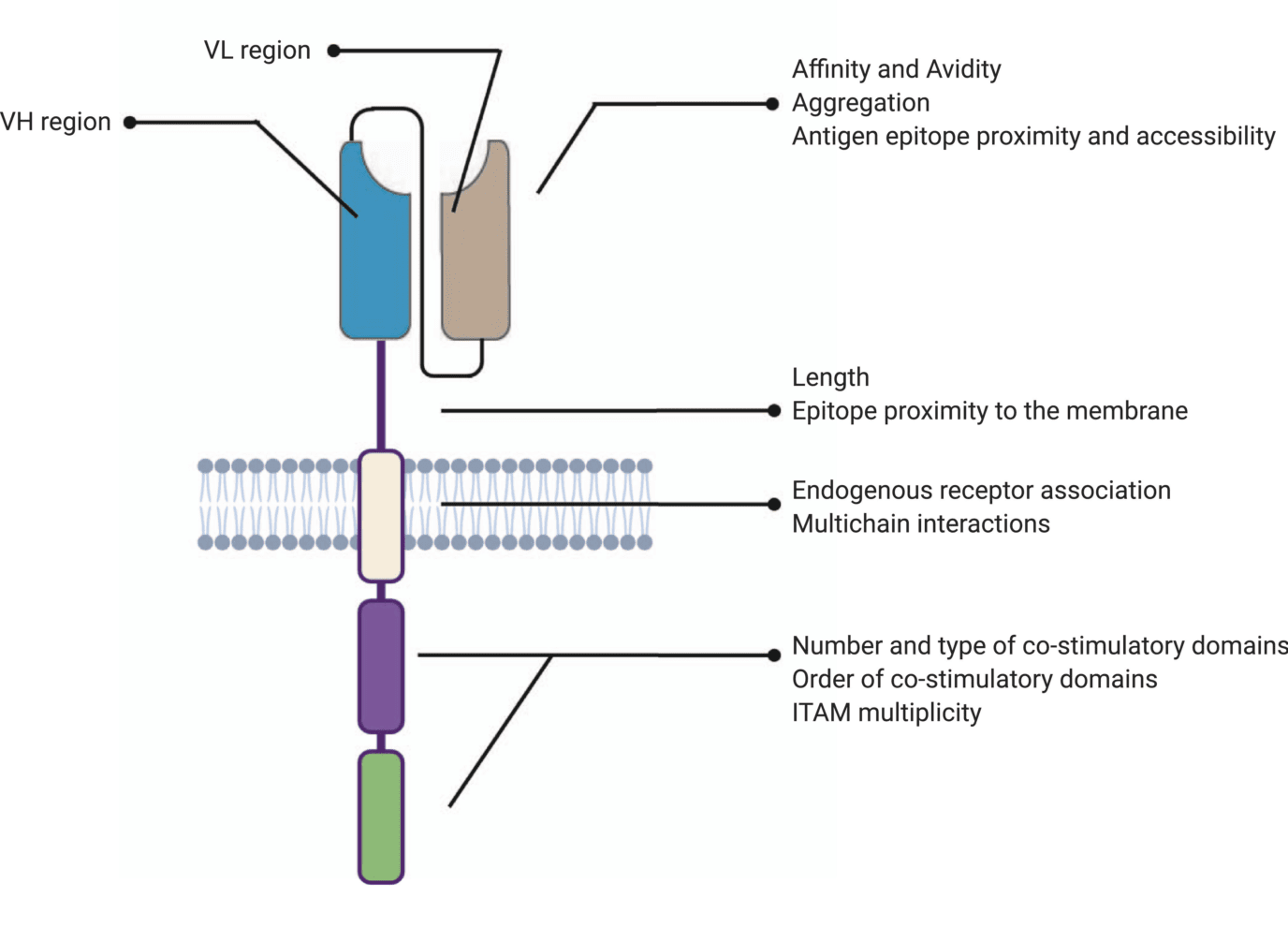
The structure of CAR-T cells
CAR-T cell therapy is an emerging alternative to conventional anticancer treatments. Over the years, these modified cells have demonstrated promising therapeutic efficacy spurning research in this field. Despite their complexity and fast evolution, CARs have maintained the same modular structure that has made them so successful in the first place. Understanding these components is the first step to designing successful CAR-T platforms.
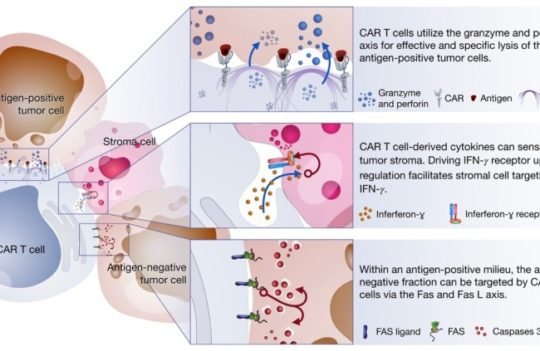
Chimeric antigen receptor (CAR)-T cells have emerged as a promising alternative to conventional radiation, chemotherapy, and immunotherapy for the treatment of malignant cancers. CARs are engineered receptors designed to modulate T cell receptors (TCRs) and consist of four main components: (i) an extracellular target antigen-binding domain, (ii) hinge or spacer region, (iii) transmembrane domain, and (iv) one or several intracellular signaling domains. These synthetic domains are subsequently packaged into viral vectors and transduced into T cells, where they redirect the immune response towards malignant cells.
Encouraging clinical trials have captured the attention of the scientific community and spurred interest in CAR research and development. As a consequence, CAR design has evolved massively since its inception but its general structure has withstood multiple trials and continues to provide an efficient framework for T-cell therapy development.
Antigen-binding domain
Similar to conventional antibodies, the antigen-binding domain is what confers target specificity to CARs. As a consequence, this domain also dictates the affinity and avidity of CAR-T cell therapies and consequently impacts their efficacy. Classically, this component of CARs derives from the variable heavy (VH) and light (VL) chains of monoclonal antibodies bound by a linker to form single-chain variable fragments (scFv).
These scFvs typically target membrane-bound surface receptors of cancer cells and lead to major histocompatibility complex (MHC)-independent T cell activation. Due to its role in cell activation, the antigen affinity must be sufficiently high and selective, but not high enough to induce cell death of CAR-expressing T cells. Moreover, it has also been shown that CAR-T cells with a similar affinity towards the same target may lead to dramatically different clinical outcomes. These differences can be explained by factors such as epitope location and antigen density on the surface of the cancer cell.
Another important consideration when it comes to antigen-binding domain development is scFv aggregation. scFvs play an important role in tonic signaling (i.e., signaling in an antigen-independent manner), for this reason, their excessive aggregation can result in high tonic signaling and subsequent early exhaustion of T cells. Aggregation can potentially be caused by low folding stabilities or exposed hydrophobic residues at the VH-VL interface. Limiting this effect starts my exerting a fine control over CAR’s expression levels in T cells.
In a nutshell, the optimal therapeutic efficiency of CAR-T cells starts by achieving a balance in terms of affinity, epitope-specificity, and CAR expression levels. This, in turn, highlights the need for extensive scFv characterization and engineering during the early stages of development.
Hinge region
The hinge or spacer region is the extracellular structure of CARs extending from the antigen-binding domain to the transmembrane domain. This region lends flexibility to the scFv and can be used to enhance the recognition of target epitopes in otherwise sterically inaccessible regions.
Hinge design, namely length, and composition, also impacts CAR expression, signaling, and strength activation outputs. Optimal spacer lengths depend on the position of the target epitope and the level of steric hindrance on the targeted cells. For instance, numerous studies have shown that long spacers allow better access to membrane-proximal epitopes or glycosylated antigens. In contrast, short hinges are more useful when targeting membrane-distal epitopes. But, in practice, spacer length needs to be tailored for each specific ligand-scFv pair. Similar to scFv affinity towards specific antigens, long spacers can produce particularly strong in vivo responses but also lead to activation-induced cell death.
Most commonly used hinge regions are derived from membrane-bound receptors (CD8 or CD28) or IgG (IgG1 or IgG4). However, IgG-derived spacers are known to interact with Fcγ receptors (FcγR), leading to the activation of the innate immune response and CAR-T cell depletion. For this reason, most IgG-derived spacers are mutated to minimize interactions with FcγR.
Besides their role in CAR signaling, hinge regions are commonly used to quantify and purify CAR-positive subsets of T cells after engineering. For this purpose, anti-Fc antibodies can be used when working with IgG-derived spacers. Moreover, Strep-tag sequences have been successfully introduced in the CAR spacer region to ensure a more efficient quantification and purification of CAR-positive cells.
Transmembrane domain
The transmembrane domain anchors the extracellular CAR domain to the T cell membrane. Significantly less literature assesses the importance and role of transmembrane domains in comparison to other modules of the CAR-T cell structure. However, recent reports have shown that the structure and composition of this domain can alter surface expression levels and dictate the membrane stability of expressed CARs. Transmembrane domains have been shown to govern other key reactions involved in CAR assembly, activation, and clustering. Moreover, this domain has been known to influence cytokine release which, if excessive, may lead to high unspecific toxicity.
As a general rule, most CARs incorporate a transmembrane sequence derived from the same protein from which the adjacent hinge or signaling domains were derived. Most of these domains are derived from CD3ζ, CD4, CD8α, or CD28 each conferring drastically different properties to CAR-T cells. For instance, CD3ζ-derived transmembrane domains result in the incorporation of CARs into endogenous TCRs (T-cell receptors), which is generally accompanied by increased T-cell activation and decreased complex stability. In contrast, CD8α or CD28-derived transmembrane domains have shown enhanced membrane stability. More research into the influence of these domains on the stability and influence in CAR-T efficacy is necessary.
Intracellular signaling domains
The intracytoplasmic or endodomain is the functional end of the receptor. While the ectodomains (antigen-binding and spacer domains) are responsible for directing the T cell towards a specific target and the transmembrane domain anchors CARs to the membrane, the role of the intracellular domains is to transmit a signal to the T cell and initiate the signaling cascade.
However, for efficient response, co-stimulatory signals are vital to sustaining the cascade. In the vast majority of FDA-approved CAR-T cells, the major signaling domain is typically derived from CD3ζ while the co-stimulatory domains (incorporated adjacent to CD3ζ) are derived from CD28 and 4-1BB (CD137). Alternative co-stimulatory signals such as ICOS, CD27, MYD88 plus CD40, and OX40 have also demonstrated efficacy in preclinical trials, but more studies are necessary to ascertain their in vivo efficacy.
Co-stimulatory domains are also known to dictate the T cell differentiation pathway, metabolic cycles, apoptosis, and activation-induced cell death. In recent times, research into functional intracellular signaling domains has gained importance among the scientific community, thus, the optimization of their design strategy is an important approach to ensure efficacy.
Concluding remarks
Although CAR-T cell therapies are complex, they are better understood when CARs are broken down and analyzed according to their fundamental structure. CARs are synthetic receptors meant to direct T cells to specific targets, for this reason, they contain an antigen-binding domain and hinge on the extracellular space, a transmembrane domain responsible for anchoring both to the cellular membrane, and an endoplasmic domain designed to trigger a signaling cascade, fundamental for CAR-T cell efficacy.
Despite recent progress in CAR-T cell design, these fundamental structural modules continue to serve as the framework upon which new therapies are built. However, the current difficulties in CAR-T cell development reveal the need for better high throughput screening tools to ensure all the structural components of these cells are perfectly optimized and adapted to each specific disease.
- Fujiwara, K. et al. Hinge and Transmembrane Domains of Chimeric Antigen Receptor Regulate Receptor Expression and Signaling Threshold. Cells. 2020; 9(5):1182. doi: 10.3390/cells9051182
- Jayaraman, J. et al. CAR-T design: Elements and their synergistic function. EBioMedicine. 2020; 58:102931. doi: 10.1016/j.ebiom.2020.102931
- Sterner, R. C. and Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021; 11(4):69. doi: 10.1038/s41408-021-00459-7
- Zhang, C. et al. Engineering CAR-T cells. Biomark Res. 2017; 5: 22. doi: 10.1186/s40364-017-0102-y