 CAR-T cell therapy
CAR-T cell therapy
CAR-T cell engineering: strategies to overcome remaining limitations
CAR-T cell engineering is an active field of research. Due to the complexity of these biotherapies, a multi-faceted approach must be used to ensure optimal clinical efficacies while minimizing toxicity. In this article, we summarize all the latest developments in cell engineering that have shown promising results in preclinical models and clinical trials. These strategies are already making CAR-T cells safer and allowing their wider adoption for the treatment of cancer.
- CAR-T cell engineering: Where to start?
- Recognition
- Trafficking
- Proliferation and persistency
- Tumor microenvironment
- Control mechanisms
- CAR-T cell engineering: a closer look at current limitations and opportunities
- Reduce antigen escape
- Improving the treatment of solid tumors
- Reducing systemic toxicity
- Concluding remarks
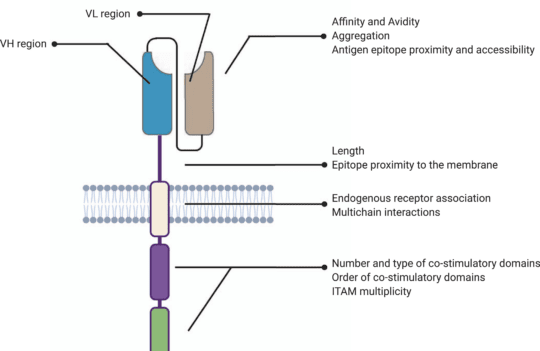
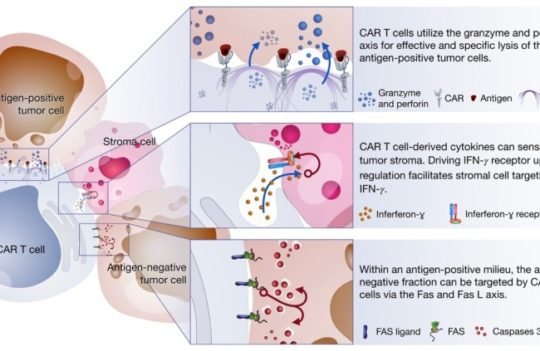
Chimeric antigen receptors (CARs) modulate natural T cell receptors (TCR) and are typically transduced/transfected into T cells sourced from autologous or allogeneic origins. This strategy has proven to be remarkably efficient at treating certain types of B cell cancers (via the CD19 antigen), namely B cell leukemia or lymphoma. CARs are characterized by an ectodomain (antigen-binding and spacer regions), a transmembrane domain, and an endodomain vital for triggering the signaling cascade upon encountering a tumor-associated antigen and subsequent cytotoxic response.
Despite the encouraging clinical success, CAR-T cells still face many challenges tied to excessive toxicity, limited anti-tumor activity, antigen escape, restricted trafficking, and reduced efficacy against solid tumors. Due to these challenges, CAR-T cell engineering is currently an active field of research where new solutions are regularly developed to improve safety, efficacy (including against solid tumors), cost, manufacturability, and availability.
CAR-T cell engineering: Where to start?
There is no simple solution to improve the efficacy of current CAR-T cell therapies. Precisely the opposite. We need multi-faceted and easy-to-validate solutions tackling several CAR-T cell limitations. At the moment, cell engineering efforts are focused on 5 aspects:
Recognition
Antigen recognition plays a huge role in CAR-T cell efficacy. For this reason, improving the specificity and selectivity of scFv domains and ensuring better access to cryptic or hidden epitopes is a vital step of cell engineering processes.
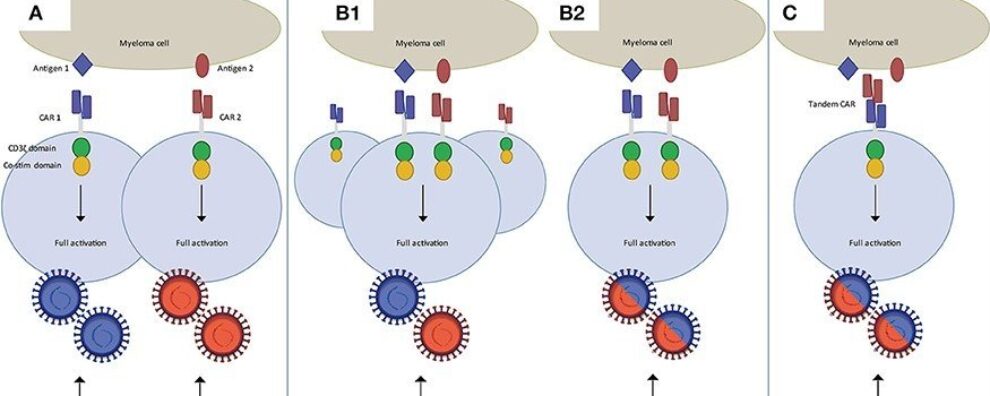
Moreover, patients who have received anti-CD19 CAR-T cell therapies showed a subsequent loss of this antigen. This antigen escape response occurs in 30-70% of the patients after treatment, indicating the need to develop CARs that target multiple antigens such as dual CARs or tandem CARs (single CAR with two scFvs).

Trafficking
Trafficking is the ability to reach tumor sites. This is not a major issue for conventional anti-CD19 CAR-T cell therapies targeting blood cancers (or liquid cancers), but it becomes a major issue when targeting solid tumors known to be more difficult to penetrate physically.

Proliferation and persistency
Proliferation is likely to dictate the clinical efficacy of CAR-T cell therapies while persistence ensures these cells survive long enough to eliminate tumors (without requiring reinfusion).
Tumor microenvironment
Many solid tumors secret a plethora of biomolecules that modulate the microenvironment, often leading to the downregulation or inhibition of T cells. For this reason, engineering CAR-T cells able to resist this suppressive pressure may be instrumental for the use of these cells against solid tumors.

Control mechanisms
CAR-T cells are known to cause, on occasion, excessive off-tumor and off-target toxicity. For this reason, engineering strategies that allow more precise control over the process in vivo could be instrumental to increase the safety of these cells. One of such strategies already in the test phase is the introduction of molecular switches. These signals allow clinicians to shut down or switch on the cytotoxic response by administering chemical signals like small drugs and thus making these therapies safer.

CAR-T cell engineering: a closer look at current limitations and opportunities
Reduce antigen escape
High relapse rates caused by tumor-associated antigen escape have led researchers to engineer dual targeting CAR-T cells. This approach has already shown success in clinical trials where dual systems such as CD19/CD22 or CD19/BCMA effectively treated diffuse large B cell lymphoma and multiple myeloma, respectively. Tandem systems where two scFv domains targeting different antigens are bound to the same receptor have also proven to be a promising strategy for dual targeting. Tandem CARs have been successful in preclinical models targeting HER2/IL13Ra2 (glioblastoma) or HER2/MUC1 (breast cancer).
In all cases, dual targeting resulted in superior anti-tumor responses compared to single-targeted CARs. However, researchers observed a significant difference in the response generated by these dual constructs, suggesting that antigen selection and optimization are crucial to prevent relapse.
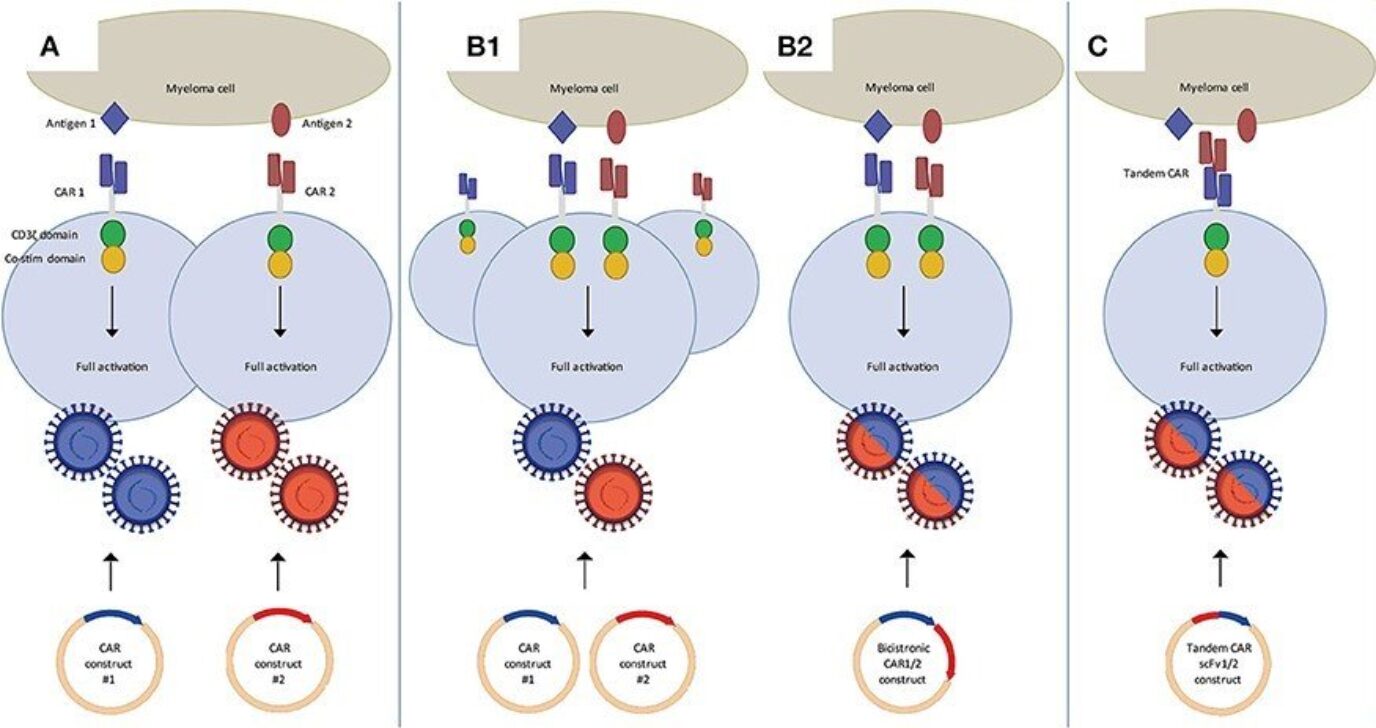
Interestingly, many tumor-associated antigens are also expressed at low levels on normal cells and tissue. For this reason, dual-targeting might not suffice to ensure CAR-T cell specificity. For this reason, researchers are tuning antigen-binding domains to recognize specific tumor-associated post-translational modifications (PTM). This strategy is useful because solid tumors are known to overexpress truncated O-glycans. In this way, scFvs that target these modified glycans are already being investigated at the preclinical stage. They are expected to further reduce the risk of antigen escape while minimizing the off-target toxicity of CAR-T cell treatments.
Improving the treatment of solid tumors
When treating solid tumors, even the combination of dual targeting and PTM-based CAR-T cell therapies may fail to produce a potent response. This recalcitrant behavior of these tumors is tied to the tumor’s extracellular matrix that works as a physical barrier. Early attempts at overcoming this barrier include the co-transfection/transduction of matrix-degrading enzymes into CAR-T cells. Enzymes that have shown promising results in preclinical models include heparanase (degrades heparin sulfate proteoglycan) and fibroblast activation protein (a serine protease that degrades tumor fibroblasts).
Besides the physical barrier, solid tumors are notorious for their immunosuppressive microenvironment. Molecules secreted by these tumors are known to downregulate T cells and thus limit CAR-T cell efficacy. One strategy to overcome this issue is the combination of CAR-T cells with immune checkpoint blockade therapies (i.e., PD-1/PD-L1 blockade). In clinical trials, the combination of the two approaches ensured a sustained CAR-T cell persistence and activity. Approved drugs for immune checkpoint blockade include nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, cemiplimab, and dostarlimab. However, more studies are needed to understand how each of these therapies interacts with CAR-T cells during treatment.
Reducing systemic toxicity
Despite the unparalleled success achieved by CAR-T cell therapies, most patients who receive this treatment still experience mild to severe systemic toxicity. These side effects result from systemic cytokine release that may result in cytokine release syndrome (CRS) associated with severe hyper inflammation, renal failure, pulmonary edema, and immune effector cell-associated neurotoxicity syndrome (ICANS) which is characterized by the disruption of the blood-brain barrier. Approved complementary therapies to manage these symptoms are often administered to CAR-T cell therapy-receiving patients – tocilizumab and corticosteroids. However, to date, no effective treatments for ICANS have been approved.
However, researchers believe that it is more important to engineer CAR-T cells in a way that reduces their inherent toxicity than to invest in symptomatic treatments. For this reason, several engineering approaches are currently under active investigation:
- Modulating CAR-T cell activation – antigen-binding affinity, epitope location, and costimulatory components play a vital role in CAR activation. For this reason, they must be optimized in tandem to ensure that the threshold for excessive cytokine production is not surpassed.
- Reducing scFv affinity towards highly expressed tumor-associated antigens – several studies have demonstrated that scFv domains with micromolar affinity are more selective for high-expression tumor antigens than scFv domains with low nanomolar/sub-nanomolar affinities. Thus, these lower affinities also reduce systemic cytokine production and CRS.
- Selection of costimulatory domains according to expected antigen density – 4-1BB costimulatory domains are known to produce less toxicity and are thus more useful to treat tumors with high antigen density. While CD28 costimulatory domains, often associated with rapid activation, are preferred for the treatment of tumors with low antigen density or when low-affinity scFvs are used.
- Reduce CAR immunogenicity – the use of human or humanized scFv fragments is known to decrease the immunogenicity of CAR-T cell therapies and subsequently improve their persistency.
- Neurotoxicity neutralization – Granulocyte-macrophage colony-stimulating factor (GM-CSF) neutralization with lenzilumab or mutational inactivation of the associated genes in CAR-T cells proven to be successful at reducing neurotoxicity and CRS. Similarly, the deletion or inhibition of tyrosine hydroxylase has also led to reduced cytokine production levels thus minimizing neurotoxicity.
- Introduction of molecular switches – molecular switches have been used successfully to modulate the activity of recombinant proteins in vivo. These strategies give us the possibility to control and arrest CAR-T cell proliferation at the onset of adverse events. Although most of them rely on suicidal mechanisms, researchers believe that temporary inhibition of activation is the best approach to allow the rescue of CAR-T cells and continue treatment when toxicities have subsided.
Another strategy being used to minimize toxicity requires a shift from systemic delivery of CAR-T cell therapies towards local administration. Multiple clinical trials have demonstrated the superior efficacy of local administration versus systemic delivery. However, this is especially relevant when treating single tumors but less effective at tackling metastatic cancers.
Concluding remarks
Antigen escape (high relapse rates), systemic toxicity, and limited efficiency against solid tumors are the major issues that have hindered the widespread adoption of CAR-T cell therapies. Solving these issues starts by recognizing that both CAR and tumor properties must be taken into account during the early development of new therapies.
Many engineering approaches have been used in recent years to increase CAR-T cells’ safety. The combination of CAR-T cell therapy with immune checkpoint blockade, the introduction of dual targeting CARs, and the inclusion of molecular switches are only a few of the approaches that might expand the current boundaries and applicability of this emerging therapy.
- Lim, W. A. and June, C. H. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017; 168(4):724-740. doi: 10.1016/j.cell.2017.01.016
- Sterner, R. C. and Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021; 11(4):69. doi: 10.1038/s41408-021-00459-7
- van der Schans, J. J. et al. Dual Targeting to Overcome Current Challenges in Multiple Myeloma CAR T-Cell Treatment. Front Oncol. 2020; 10:1362. doi: 10.3389/fonc.2020.01362