 Peptide synthesis
Peptide synthesis
Coronavirus: lessons from the hunt for a universal flu vaccine
More than a century ago, the world faced the most devastating pandemics in history – the Spanish flu. Caused by an unusually deadly strain of influenza (H1N1 flu virus), the pandemic swept a world weakened by war and famine causing the deaths of potentially 50 million people. Today, we are facing a new deadly pandemic caused by an atypical strain of coronavirus – SARS-CoV-2. But while we watch closely the troubling numbers of the new crisis, seasonal influenza continues to take a heavy toll behind the scenes, urging us to keep looking for a long-term universal flu vaccine.
Lessons from influenza and the need for a universal flu vaccine
According to the World Health Organization (WHO), between 290 and 650 thousand people die each year from influenza-related respiratory illnesses around the world. Despite recent breakthroughs in therapeutics and prophylactic vaccination, this worrisome trend has not shown signs of improvement.
Currently, 4 different types of influenza have been identified: A, B, C, and D. Despite the diversity, only types A and B were found to be responsible for most influenza-related illnesses in humans. Influenza strains of type A, for instance, can infect humans or animals. Occasionally, a zoonotic strain may jump between species and develop sustained human-to-human transmission leading to a pandemic. On the contrary, influenza type B circulates among humans and seals causing what we know as the seasonal flu. Unlike type A, type B influenza has not been shown to cause pandemics, presumably due to its limited host range and reduced chances for reassortment (swapping of genes between different strains of the virus).
There is compelling evidence supporting vaccination as the best strategy to control the influenza epidemic. But the creation of a universal flu vaccine against this fast-mutating pathogen still faces many challenges.
Typical seasonal vaccines against influenza target the viral hemagglutinin (HA) surface protein. HA is one of the major surface proteins across different types and strains of influenza. Furthermore, it’s responsible for binding the host’s receptors and mediating viral entry. Antibodies that bind strain-specific HA can block the virus entry and prevent widespread infection.
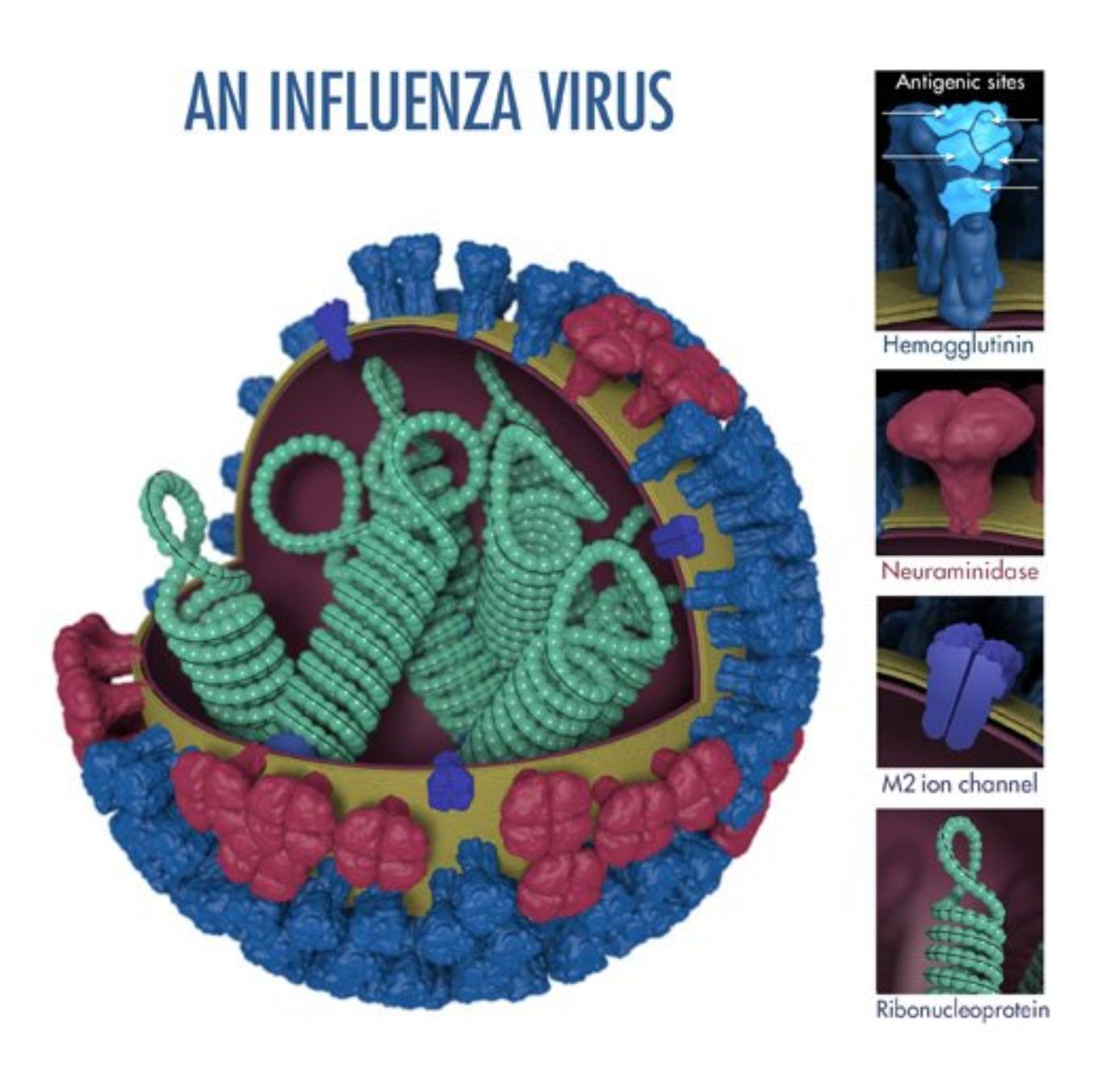
However, the replication of this virus is mediated by an error-prone RNA polymerase, explaining its unusually high mutation rate. Due to the lack of proofreading by the RNA polymerase allied to the positive selection for escape mutants by pre-existing neutralizing antibodies in the population, the composition of the seasonal vaccine must be reevaluated and reformulated twice per year – once for each hemisphere. Researchers need to anticipate the antigenic drift of circulating strains at least 7-8 months before the flu season to produce the best-adapted vaccine.
Besides the technical constraints associated with annual reformulation, the seasonal approach does not guarantee protection against antigenically divergent seasonal strains and pandemic strains of the virus, which are extremely hard to predict. Moreover, the conventional seasonal approach requires annual vaccination and often fails to elicit a protective immune response in the elderly (one of the most important risk groups) due to the decline of their immune system (immunosenescence).
Universal flu vaccines under clinical evaluation
Although seasonal vaccination has proven to produce a protective response against many strains in the adult population, researchers believe there is a better way to fight influenza. The search for a better way has become increasingly urgent due to the devastating effects of recent seasonal outbreaks and the recurrent emergence of pandemic strains of the virus.
The shortcomings of seasonal vaccination tell us that we still need a vaccine able to produce broad and durable protection even against pandemic strains of influenza, in other words, we still need a universal flu vaccine.
Over the decades, the hunt for the universal vaccine has focused on producing a protective response by targeting influenza’s conserved proteins or conserved domains within highly variable proteins. However, an inherent obstacle to the use of conserved domains/proteins is that these often have reduced inherent immunogenicity and may lead to a weak immune response. But in recent years, some researchers have made astounding progress and the first universal flu vaccine may reach the market in the next couple of years.
The leading candidates for the universal vaccine are:
Multimeric-001 (M-001)
Originally devised by researchers from the Weizmann Institute of Science and developed by BiondVax, M-001 has completed phase II clinical trials and its currently initiating phase III. Unlike conventional seasonal vaccines targeting the major surface protein (HA) and producing a narrow-range humoral response, M-001 was designed to leverage both the humoral and the cell-mediated immune response.
Why leveraging the cell-mediated response might work? Several studies have proven that cytotoxic T cells can kill and remove influenza-infected cells. Researchers identified internal viral proteins PB1-PB2-PA (the three subunits of influenza’s RNA polymerase), NP (nucleoprotein), and M2 and M1 (matrix proteins), as the triggers of that response.
M-001 consists of a recombinant multimeric protein containing the fusion of specific conserved and common regions to types A and B of HA, NP (nucleoprotein), and M1 (matrix protein). In preclinical studies, the construct produced an adequate immune response in B cells, helper T cells (associated with strong and long-lasting protection), and cytotoxic T cells. Results from phase I and II clinical trials further indicate that the new vaccine provides a broad protective response against seasonal and pandemic strains of influenza, across multiple age groups (from younger adults to the elderly population, 65+) without an adjuvant.
Moreover, synergistic effects were observed when the prophylactic treatment was administered in conjugation with the seasonal flu vaccine. At a potential price per dose of $10 to $50, M-001 could be administered to the entire population every 3-5 years to protect against seasonal and pandemic strains of influenza.
Flu-v
Flu-v is a peptide vaccine developed by PepTcell. It aims at providing broad immunity against types A and B of influenza by triggering the cell-mediated immune response. Unlike M-001, Flu-v was designed to incorporate a mixture of peptides corresponding to the conserved domains of several internal non-structural proteins. In doing so, the developers hope that this new vaccine construct will allow viral clearance by stimulating the release of pro-inflammatory cytokines, perforin, and granzyme by cytotoxic T cells without relying on antibody-mediated viral elimination.
The new vaccine has already demonstrated its efficiency, safety, and immunogenicity in animals. Adjuvanted Flu-v administered as a single dose or as a two-dose regimen has been evaluated in healthy human volunteers. The clinical trial showed that the formulation elicited an adequate cytotoxic T cell activation in a single-dose regimen, reducing the severity of the illness in healthy individuals.
Nevertheless, more studies are necessary to understand how the elderly respond to the vaccine and to determine if regular doses are necessary to maintain the cell-mediated protection against a broad range of influenza strains.
H1ssF_3928
H1ssF_3928 is a nanoparticle-based vaccine displaying an important epitope of influenza on its surface. The nanoparticle, made of nonhuman ferritin – a natural and degradable protein, is designed to display HA spikes in its surface, mimicking the natural organization of influenza.
But unlike conventional seasonal flu vaccines that target the head region of HA, H1ssF_3928 has been designed to target the stem of this important structural protein. Contrary to the head, the stem region of HA was found to be less variable among different types and strains of influenza. This makes it a more attractive target for a universal flu vaccine. Unlike the previous two candidates, this vaccine relies solely on the humoral response for viral clearance. Although its safety has been shown in phase I clinical trials, more studies are necessary to understand if the new construct provides long-lasting and broad protection against different strains of influenza.
Lessons from influenza and the coronavirus pandemic
Over the years, efforts to develop a universal flu vaccine taught us that targeting conserved domains may be a better solution than targeting the highly variable and strain-specific regions of a new pathogen. But why is this relevant in the fight against COVID-19?
SARS-CoV-2 is the third strain of coronavirus from a zoonotic origin to develop a sustained human-to-human transmission. However, significant differences were found in the major surface protein between this strain and previously described SARS-CoV responsible for the 2003 outbreak. Moreover, recent studies showed that our organism’s humoral response to this surface protein that mediates viral entry (spike glycoprotein S) is specific to the new strain and fails to cross-react with other strains of coronavirus.
Although the results are preliminary, they show us that the highly variable region of the spike may serve as a poor target for broad-spectrum protection against coronavirus. Also considering that vaccine development is a lengthy process, a SARS-CoV-2 strain-specific vaccine may arrive too late to help us fight the current pandemic.
The decades-long fight against influenza, taught us that focusing on the conserved regions of virus proteins may help us achieve broader and more long-lasting protection against different strains of the same pathogen. As Professor Ruth Arnon (Weizmann Institute of Science), the original developer of the M-001 universal vaccine, said in a recent interview: “If I were working in the coronavirus field, this non-changing protein approach is where I would focus my efforts.”
Concluding remarks
The first universal flu vaccine may reach the market during the next couple of years. Unlike conventional seasonal vaccination, candidates for the universal vaccine rely on targeting the conserved regions of the influenza virus. Moreover, two of the lead candidates (M-001 and Flu-v) rely on cell-mediated response to improve viral clearance.
Vaccines are easily produced but their clinical evaluation is a lengthy and uncertain process. Our experience in fighting influenza-mediated pandemics taught us that strain-specific vaccines often arrive too late.
For this reason, experts believe that the fight against pandemic strains of fast-mutating viruses should also rely on targeting the conserved regions between different strains of the virus. The race to develop strain-specific COVID-19 vaccines and the efforts to develop a universal flu vaccine will tell us which of these approaches will be more effective in the long-term.
- Atsmon, J. et al. Safety and immunogenicity of multimeric-001–a novel universal influenza vaccine. J Clin Immunol. 2012; 32(3):595-603. doi: 10.1007/s10875-011-9632-5
- Houser, K. and Subbarao, K. Influenza Vaccines: Challenges and Solutions. Cell Host Microbe. 2015; 17(3): 295–300. doi: 10.1016/j.chom.2015.02.012
- Ju, B. et al. Potent human neutralizing antibodies elicited by SARS-CoV-2 infection. bioRxiv. doi: 10.1101/2020.03.21.990770
- NIH Begins First-in-Human Trial of a Universal Influenza Vaccine Candidate. Retrieved from https://www.niaid.nih.gov/news-events/nih-begins-first-human-trial-universal-influenza-vaccine-candidate. Accessed on April 2020
- Pleguezuelos, O. et al. Efficacy of FLU-v, a broad-spectrum influenza vaccine, in a randomized phase IIb human influenza challenge study. NPJ Vaccines. 2020 Mar 13; 5:22. doi: 10.1038/s41541-020-0174-9
- Roser, M. The Spanish flu (1918-20): The global impact of the largest influenza pandemic in history. Our World in Data. March 4, 2020. Retrieved from https://ourworldindata.org/spanish-flu-largest-influenza-pandemic-in-history
- Schultz-Cherry, S. Is It Possible? A Different Approach to Creating a Universal Influenza Vaccine. mBio. 2015; 6(5): e01580-15. doi: 10.1128/mBio.01580-15
- van Doorn, E. et al. Evaluating the immunogenicity and safety of a BiondVax-developed universal influenza vaccine (Multimeric-001) either as a standalone vaccine or as a primer to H5N1 influenza vaccine: Phase IIb study protocol. Medicine (Baltimore). 2017; 96(11):e6339. doi: 10.1097/MD.0000000000006339
- Weizmann Institute of Science. Prof. Ruth Arnon: Lessons from influenza. Retrieved from http://www.weizmann.ac.il/WeizmannCompass/sections/briefs/prof-ruth-arnon-lessons-from-influenza Accessed on April 2020