 Antibody production
Antibody production
Use recombinant antibody expression to unlock the full potential of fusion proteins
Antibodies are essential tools for modern medicine and diagnostics. Interestingly, many researchers believe that we are only now beginning to grasp the full potential of these molecules. In this context, recombinant antibody expression technologies have allowed the production of complex antibody constructs. Currently, the recombinant antibody production of alternative formats such as Fc-fusion proteins is one of the most promising ways to rethink the potential of antibodies for numerous applications.
The revolution of recombinant antibody expression technology
Murine monoclonal antibodies may have been the first type of immunomolecules ever to gain FDA approval for clinical use. But, from a statistical point of view, they found limited application in clinical settings.
Their limitations are tied to the fact that most IgG-like monoclonal antibodies have limited diffusion across tissues. Moreover, non-human hosts generate antibodies with low compatibility with the human organisms and, thus, cause allergic responses.
But many solutions have been proposed and explored since the discovery of these antibodies and their inherent limitations. Most of these solutions consist in using these antibodies as frameworks to develop more effective biotherapeutic molecules. For instance, antibody sequences can be used and engineered for the production of antibody fragments or fusion constructs.
All of these approaches were only made possible by the onset of recombinant antibody expression techniques.
These techniques, in combination with transient expression in mammalian expression vectors, have allowed the development of antibody fusion constructs with human-like glycosylation.
Recombinant antibody expression as fusion constructs has been a popular approach to simplify antibody engineering. Traditionally, the conjugation and fragmentation of antibodies happened in the post-production stage. However, the process was found to be inefficient and to lead to the generation of heterogeneous populations due to incomplete fragmentation or non-specific labeling.
In recent years, much research has become available on the development of these antibody constructs, and the benefits of using these strategies to improve the efficiency of natural antibodies. One of the most popular types of constructs are Fc-fusion proteins either in monomeric or polymeric form.
Fc-fusion proteins for therapeutic application
Fc-based fusion proteins consist of a hybrid protein containing a conserved domain Fc of an antibody directly fused to a peptide or protein with therapeutic value. The most marked benefit of fusing a protein to a functional Fc domain is the extension of the protein’s half-life in patient’s plasma.
The half-life of these constructs in typically shorter than that of intact monoclonal antibodies (1-2 weeks against 3-4 weeks, respectively). However, they are still significantly longer than the half-life of peptide and protein drugs alone (few minutes or hours).
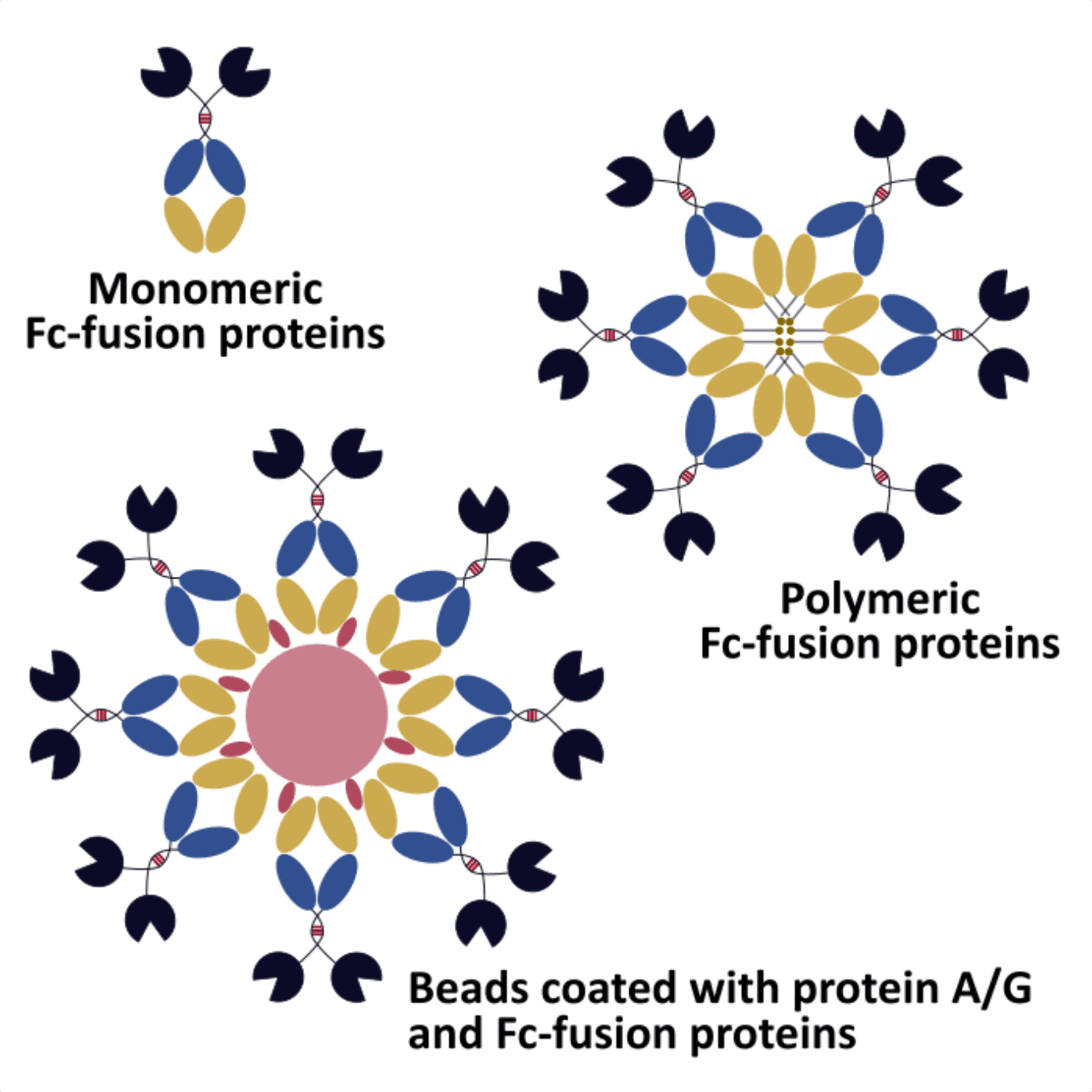
The Fc domain also allows these molecules to interact with Fc-receptors, which makes them extremely useful as anticancer agents and vaccines. From a technical point of view, the presence of this domain can improve the stability and solubility of peptides and proteins, which otherwise would be quickly degraded by widespread proteases.
Moreover, the recombinant expression of these constructs, allows the easy and cost-effective purification of these hybrid molecules using protein A/G affinity chromatography, a method that is also commonly used to purify polyclonal antibodies from serum samples.
Interestingly, Fc-fusion protein constructs are not a new concept. The first construct of this type ever to be approved was Etanercept (Drug Bank accession no. DB00005) for the treatment of rheumatoid arthritis. The construct, approved in 1998, consists of 934 amino acids and it is recurrently expressed in Chinese hamster ovary (CHO) expression systems.
In 2016, Etanercept was still among the top 3 best-selling drugs worldwide. Moreover, up to that date, Fc-proteins represented 20% of all antibody-based therapies approved by the FDA.
But how do these constructs act on our organism?
Fc-proteins are designed to target receptor-ligand interactions. They are usually produced and administered in monomeric form, presumably due to the low diffusion rate of polymeric constructs. For instance, they can act as antagonists blocking specific receptors (e.g. Etanercept is an antagonist drug), as agonists stimulating receptor functions or by enhancing a specific immune response in a patient.
| Name (commercial designation) | Description | Indication | Year of FDA approval |
|---|---|---|---|
| Asfotase alfa (Strensiq) | TNSALP – Fc fusion-peptide | Hypophosphatasia | 2015 |
| Dulaglutide (Trulicity) | GLP-1 – Fc fusion | Type 2 diabetes | 2014 |
| Efmoroctocog alfa (Eloctate) | Monomeric Fc domain-deleted F-VIII fusion | Hemophilia A | 2014 |
| Eftrenonacog alfa (Alprolix) | Monomeric Factor IX Fc fusion protein | Hemophilia B | 2014 |
| Ziv-aflibercept (Zaltrap) | VEGFR-Fc fusion protein Trap | Metastatic colorectal cancer | 2012 |
| Belatacept (Nulojix) | CTLA-4 fused to the Fc of human IgG1 | Organ rejection | 2011 |
| Aflibercept (Eylea) | VEGFR1/VEGFR2 fused to the Fc of human IgG1 | Age related macular degeneration | 2011 |
| Rilonacept (Arcalyst) | IL-1R fused to the Fc of human IgG1 | Cryopyrin-associated periodic syndromes | 2008 |
| Romiplostim (NPlate) | Thrombopoietin-binding peptide fused to the Fc of human IgG1 | Thrombocytopenia in chronic immune thrombocytopenic purpura patients | 2008 |
| Abatacept (Orencia) | Mutated CTLA-4 fused to the Fc of human IgG1 | Rheumatoid arthritis | 2005 |
| Alefacept (Amevive) | LFA-3 fused to the Fc of human IgG1 | Psoriasis and transplant rejection | 2003 |
| Etanercept (Enbrel) | TNFR fused to the Fc of human IgG1 | Rheumatoid arthritis | 1998 |
The most interesting property of Fc-proteins is their ability to regulate effector function.
For some pathologies, such as cancer, it is important to devise techniques that can stimulate the effector function by improving the interaction with natural killer (NK) cells, monocytes or macrophages.
Some studies showed that these functions could be enhanced by modifications of the amino acid sequence of the Fc domain or by de-fucosylation of the N-linked oligosaccharides in the same region.
For other pathologies, such as the ones that require the neutralization of cytokines, it is best to repress the effector function. This can be done by using the human Fc region from human IgG2 and IgG4, which have lower affinity than IgG1-subclass antibodies towards complement receptors.
The benefits of using IgG2/4 constructs has been already demonstrated by the development of eculizumab (Soliris®, DrugBank accession no. DB01257). This monoclonal antibody consists of a junction of CH1 regions of human IgG2 and CH2 and CH3 regions of human IgG4, and it is recurrently used to treat autoimmune conditions.
However, to date no Fc construct containing IgG2/4 regions has reached the clinic. Although several of these molecules are currently under development.
One example is the fused protein containing glucagon like peptide 1 (GLP-1) and human IgG2. The construct showed superior therapeutic and pharmacological properties when compared to native GLP-1 for the treatment of type I diabetes in a mouse model. But this hybrid molecule has yet to pass preclinical and clinical trials.
Unfortunately, none of the Fc constructs developed to date have succeeded in activating the complement cascade that can trigger cell death by lysis. This is presumably due to the lack of a functional Fab residue. Therefore, the development of Fc constructs with cytotoxic ability will probably require the modification of the Fc framework to compensate the lack of a Fab region.
Fc-fusion constructs as vaccines
There is a general feeling among the scientific community that the potential of this type of constructs are only now starting to be truly understood. It is estimated that the most exciting progresses will come from the development of Fc-fusion proteins that can serve as vaccines.
But, up to now, despite the number of publications reporting the success of this type of constructs for immunization in animal models, none of these molecules has excelled at preclinical and clinical trials.
Nevertheless, Fc-fusion proteins have considerable potential from a safety and immunogenic point of view. For this reason, researchers are persisting on the development of constructs for this specific application.
The rational behind this strategy is the development of vaccines with Fc regions that target antigen-presenting cells (APCs). This could lead to an enhanced antigen uptake and processing. At the same time, the presence of this domain, especially in a polymeric form can also reduce the need for co-administration with an adjuvant, which sometimes is responsible of causing undesirable allergic reactions.
As of 2019, the Fc fusion protein vaccine closest to get FDA approval is the investigational V920 Ebola vaccine developed by Merck. The vaccine, initially engineered by researchers from the Public Health Agency of Canada’s National Microbiology Laboratory, consists of an extracellular domain of the Zaire Ebola virus (ZEBOV) GP fused to a Fc domain of a human IgG1 subclass antibody. The vaccine has excelled during phase I/II clinical trials and it is likely to receive approval during 2020.
Fc-fusion constructs for non-clinical applications
The stability of Fc-fusion proteins has also made them desirable reagents for several non-clinical applications including flow cytometry, immunohistochemistry and protein microarrays.
The advantages of using a Fc domain in this type of assays are similar to the ones that have first sparked the interest in these molecules for clinical applications:
- Greater protein stability with reduced risk of loss of affinity towards a specific binder
- Possibility to conjugate these molecules to surface-bound or bead-bound protein A/G, which can be explored for high throughput approaches
The attachment of these constructs to a surface has been successfully used to study protein-protein interactions. Moreover, this technique revealed to be especially sensitive to detect low affinity interactions, often unnoticed in mass spectrometry studies.
Microarray formats were also found to be useful for the detection of autoantibodies linked to important autoimmune diseases. However, the full potential of these reagents remains to be explored as more studies are necessary to further develop these techniques.
Concluding remarks
Recombinant antibody expression technologies have led to the development of many different antibody formats. In this context, the development of Fc-fusion proteins gave rise to the most promising results.
Fc-proteins in monomeric, polymeric or surface-bound formats have found numerous applications. However, the most significant results were obtained in the development of Fc-protein drugs to combat different pathologies.
More recently, exciting breakthroughs have been described for Fc protein vaccines. After almost 10 years of development and preclinical and clinical studies, V920, an Ebola targeting vaccine, may finally get FDA approval during 2020.
- Czajkowsky, D. M. et al. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med. 2012; 4(10): 1015–1028. doi: 10.1002/emmm.201201379
- Kim, M. Y. et al. Plant‐expressed Fc‐fusion protein tetravalent dengue vaccine with inherent adjuvant properties. Plant Biotechnol J. 2018; 16(7):1283-1294. doi: 10.1111/pbi.12869
- Monath, T. P. et al. rVSVΔG-ZEBOV-GP (also designated V920) recombinant vesicular stomatitis virus pseudotyped with Ebola Zaire Glycoprotein: Standardized template with key considerations for a risk/benefit assessment. Vaccine X. 2019; 1: 100009. doi: 10.1016/j.jvacx.2019.100009
- Rohrbach, P. et al. Therapeutic antibodies and antibody fusion proteins. Biotechnol Genet Eng Rev. 2003; 20:137-163. doi: 10.1080/02648725.2003.10648041
- Rosenberg, J. M. et al. Protein Microarrays: A New Tool for the Study of Autoantibodies in Immunodeficiency. Front Immunol. 2015; 6: 138. doi: 10.3389/fimmu.2015.00138
- Strohl, W. R. Current progress in innovative engineered antibodies. Protein Cell. 2018; 9(1): 86–120. doi: 10.1007/s13238-017-0457-8
- Werle, M. and Bernkop-Schnürch, A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006; 30(4):351-367. doi: 10.1007/s00726-005-0289-3




