 Custom assay
Custom assay
What types of antibody neutralization tests are currently used in vaccine and drug development?
Measuring the neutralizing response has become vital for the correct assessment of the efficiency of new vaccines and drugs. But conventional in vitro neutralization tests can be labor-intensive, time-consuming, and require access to biosafety level 3 (BSL3). The COVID-19 pandemic has fostered the quick development of alternative tests to monitor the development of this response. What are the main advantages of these tests in comparison to the most conventional methods?
- How neutralization tests can be used to boost antibody development
- Neutralizing tests for COVID-19 drug and vaccine development
- Conventional and high-throughput antibody neutralization tests
- Plaque-reduction neutralization test (PRNT)
- Pseudovirus neutralization test (pVNT)
- Surrogate Virus Neutralization Test (sVNT)
- ProteoGenix’s neutralization test for fast screening of candidate therapeutic antibodies
- Concluding remarks
How neutralization tests can be used to boost antibody development
Neutralization can be defined as the functional blocking of cell surface-expressed proteins by specific antibodies. These antibodies, named neutralizing antibodies (NAbs), play a vital role in the antiviral response and prevent viral entry by:
- Blocking receptor engagement
- Arresting the membrane fusion process
- Accelerating the decay of viral particles
Moreover, NAbs can promote the quick elimination of pathogenic viruses from the organism without the need to further engage the patient’s immune system.
Today, the ability to quickly monitor the neutralizing response became crucial for the development of efficient vaccines and drugs. This response can be measured with the aid of different types of in vitro tests which are now recurrently employed for fast screening in long-term vaccine surveillance studies, candidate vaccine clinical development, and therapeutic antibody development.
Neutralizing tests for COVID-19 drug and vaccine development
The COVID-19 pandemic continues to take a heavy toll and impose a heavy burden on our healthcare systems. Today, it is more important than ever to develop in vitro neutralization tests that are amenable to standardization, parallelization, and miniaturization while maintaining high accuracy and sensitivity.
In SARS-CoV-2, it is well-established that the spike glycoprotein plays a vital role in the infection cycle. The spike binds the human cell surface receptor ACE2 via its C-terminal domain (S1 subunit) which contains the receptor-binding domain (RBD). This interaction drives the proteolytic cleavage of the spike exposing the N-terminal domain (S2 domain). This domain is then responsible for catalyzing the fusion between the viral and human cell membranes.
COVID-19 drug and vaccine development has mostly focused on targeting S1 or RBD. This choice allowed the quick adaptation of multiple neutralizing test formats to accelerate vaccine and drug development.
Conventional and high-throughput antibody neutralization tests
Plaque-reduction neutralization test (PRNT)
The conventional approach to measuring neutralizing antibody activity is the plaque-reduction neutralization test (PRNT). It allows the detection and quantification of virus-specific neutralizing antibodies. However, despite being the most robust in vitro neutralization test, it also suffers from considerable drawbacks including its cost and the need to access biosafety level 3 (BSL3) facilities when working with pathogenic viruses.
This neutralization test, first developed by Henderson and Taylor in the late 1950s, requires the use of native viral particles as well as virus-susceptible cells. Initially, the virus and a sample (serum, plasma, monoclonal or polyclonal antibodies) are mixed allowing the formation of virus-antibody complexes. Subsequently, the mixture is added to the virus-susceptible cells which are then cultured in semi-solid media and incubated until plaque formation is observed (typically between days 3 and 5).
Dyes are used for revealing plaques during or after incubation and negative controls (antibody-negative serum or non-neutralizing antibodies) are used to assess the test’s accuracy. Since virus concentration is kept constant and different dilutions of the sample are tested, neutralizing antibody titers can be easily determined. Several formats and variations exist to the classic PRNT, including the use of fluorescence-tagged viral particles allowing the fast measuring of neutralizing activity.
Due to its versatility, this test is extremely useful in vaccine surveillance studies or during clinical development of new vaccine formulations, but due to being time-consuming and labor-intensive, it has found limited application in therapeutic antibody development.
Pseudovirus neutralization test (pVNT)
To overcome the hurdles imposed by the classical plaque-reduction neutralization test, researchers have begun developing alternative test formats. Pseudovirus neutralization tests (pVNT) are one of the most popular alternatives. These tests are based on non-infectious viral particles engineered to integrate an exogenous surface protein.
The resulting pseudotyped viral particles thus integrate the surface protein of a highly infectious virus without retaining its pathogenic properties. In this way, neutralization tests can be safely performed in laboratories equipped for biosafety level 2 (BSL2) experiments and can also be easily implemented for large-scale testing. Neutralization tests with the pseudotyped virus are then performed in the classic plaque-reduction format.
Several challenges arise when developing this type of test for measuring neutralizing activity. For instance, since the test is virus-specific it is necessary to develop new pseudovirus for each new pathogenic viral strain. Moreover, the engineered chimeric strain needs to be optimized in regards to surface protein density and structure, to ensure the chimeric constructs correctly mimic the interaction between the native virus and its target and retains the original infectivity.
This adds complexity to the development stage making it time-consuming and labor-intensive. Moreover, since pVNT remains a culture-dependent approach to measuring neutralizing activity, it is still necessary to incubate the samples for some days (3-5) to determine the results.
Surrogate Virus Neutralization Test (sVNT)
Surrogate neutralization tests (sVNT) are the most flexible type of neutralization test. It is a culture-independent approach that can be easily adapted to the popular ELISA (enzyme-linked immunosorbent assay) format. Furthermore, it is based on the use of specific protein domains involved in receptor binding and thus forgoes the need to use the active virus or cells for screening neutralizing activity.
This type of neutralization tests has shown a high specificity in detecting the presence of neutralizing antibodies in HIV-infected patients. Some of the unique advantages of this test format include:
- Easy adaptation to high-throughput screening
- Results are obtained in hours instead of days
- Tests can be performed without the need for special biosafety facilities or specialized equipment
Although these tests cannot replace pVNT or PRNT, they can greatly assist in the fast screening of samples or purified antibodies for neutralizing activity.
ProteoGenix’s neutralization test for fast screening of candidate therapeutic antibodies
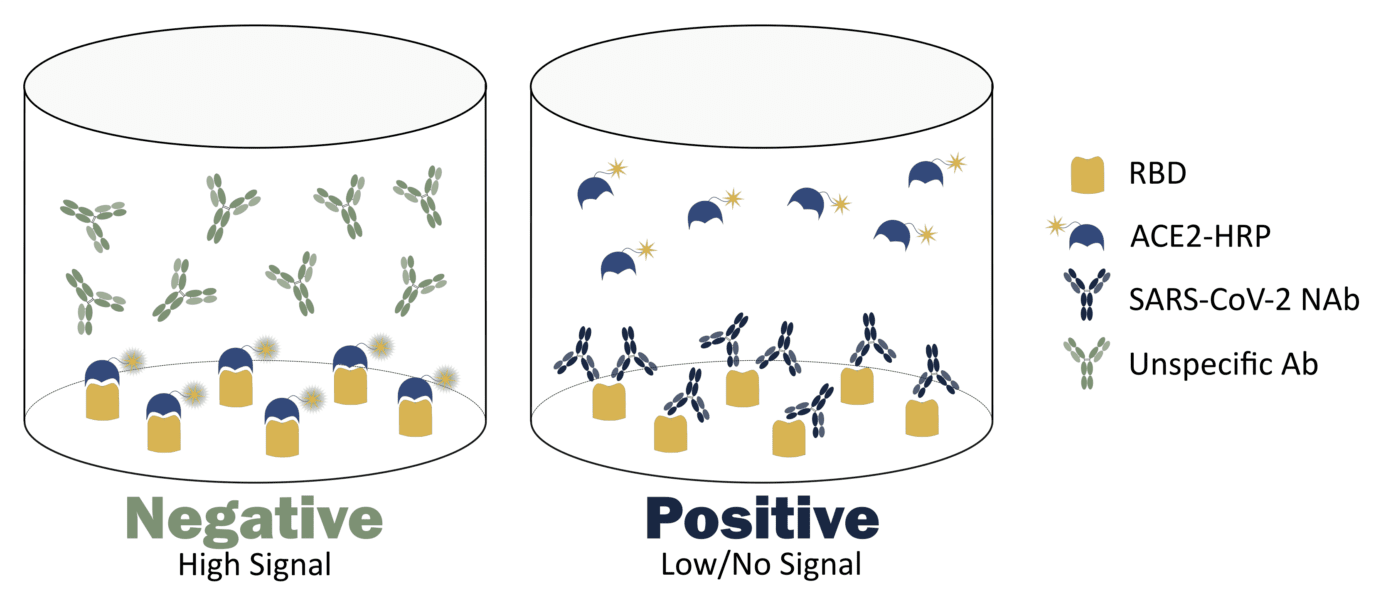
At ProteoGenix, we have recently created a surrogate neutralization test to boost the development of anti-SARS-CoV-2 antibodies. It makes use of immobilized SARS-CoV-2 RBD and HRP-tagged human ACE2 to perform the indirect detection of neutralizing antibodies.
The test is based on the principle of competitive ELISA, where the antibodies present in specific samples compete with HRP-tagged ACE2 for the binding pocket of RBD. Due to its indirect and competitive format, the test can be interpreted as follows:
- In the presence of an unspecific antibody, the HRP-tagged ACE2 will bind to the immobilized RBD and produce a strong signal when the 3,3′,5,5′-tetramethylbenzidine (TMB) substrate is added to the well
- In the presence of a neutralizing antibody, the antibody will bind to the immobilized RBD and thus block the interaction between this domain and the HRP-tagged ACE2. As a result, when the TMP substrate is added to the medium, no signal (or low signal) will be produced
Concluding remarks
Different tests are currently available to measure neutralizing activity in complex or purified samples. Among these tests, the plaque-reduction neutralization test is often referred to as the gold standard for in vitro detection. However, despite being one of the most reliable tests currently available, it still suffers from considerable drawbacks including the need to use native viral particles, virus-susceptible cells, and access to biosafety level 3 (BSL3) facilities.
These characteristics make the test hard to scale and impossible to adapt to high-throughput screening. Pseudoviruses partially solved this problem by creating surrogate chimeric viruses that maintain the original infectivity without retaining pathogenicity. This format makes it more amenable to large-scale testing but does not shorten lead times.
Surrogate virus neutralization tests (sVNT) have recently arisen as a suitable alternative to conventional tests for the high-throughput screening of neutralizing activity. It simplifies the test by using only the specific protein domains involved in receptor binding and it forgoes the need to access special facilities (BSL3) and considerably shortens lead times from a few days to only a few hours. Although these tests cannot fully replace conventional neutralization testing, they can accelerate the efforts in vaccine, drug, and diagnostic tools development.
- Henderson, J. R. and Taylor, R. M. Arthropod-borne virus plaques in agar overlaid tube cultures. Proc Soc Exp Biol Med. 1959; 101(2):257-259. doi: 10.3181/00379727-101-24902
- Nie, J. et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect. 2020; 9(1): 680–686. doi: 10.1080/22221751.2020.1743767
- Qiu, C. et al. Safe Pseudovirus-based Assay for Neutralization Antibodies against Influenza A(H7N9) Virus. Emerg Infect Dis. 2013; 19(10): 1685–1687. doi: 10.3201/eid1910.130728
- Sanchez, A. et al. Development and studies of the anti-R7V neutralizing antibody ELISA test: A new serological test for HIV seropositive patients. J Immunol Methods. 2008; 332(1-2): 53-60. doi: 10.1016/j.jim.2007.12.010
- Schmidt, F. et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med. 2020; 217 (11): e20201181. doi: 10.1084/jem.20201181

