 Antibody production
Antibody production
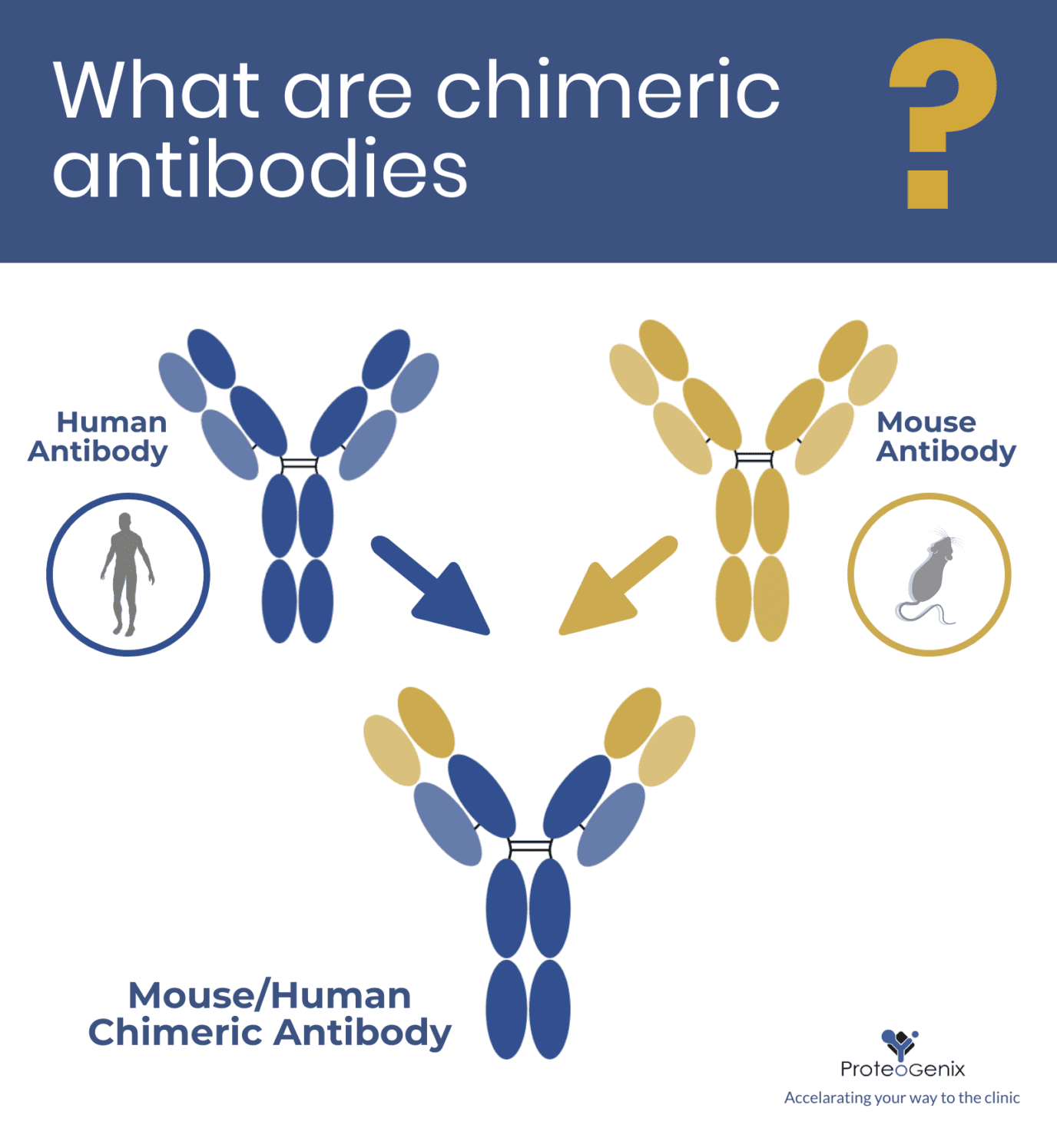
What are chimeric antibodies?
Considered by many researchers as intermediate molecules, chimeric antibody constructs have been gradually losing ground to more effective molecules such as humanized and human antibodies. Today, these structural chimeras represent only 10% of all antibodies approved for clinical use. Nevertheless, the production of these constructs continues to serve as intermediates in antibody humanization processes and, occasionally, as controls and calibrators for immunoassays in research and diagnostics.
What are chimeric antibodies and how they produced
Chimeric antibodies can be easily created by fusing the variable domain of an antibody from one host species (e.g. mouse, rabbit, llama, etc.) with the constant domain of an antibody from a different species (e.g. human). These structural chimeras were first developed to minimize the human anti-mouse antibody (HAMA) response, which compromised the efficiency of antibody treatment when xenogeneic molecules were administered to human patients.
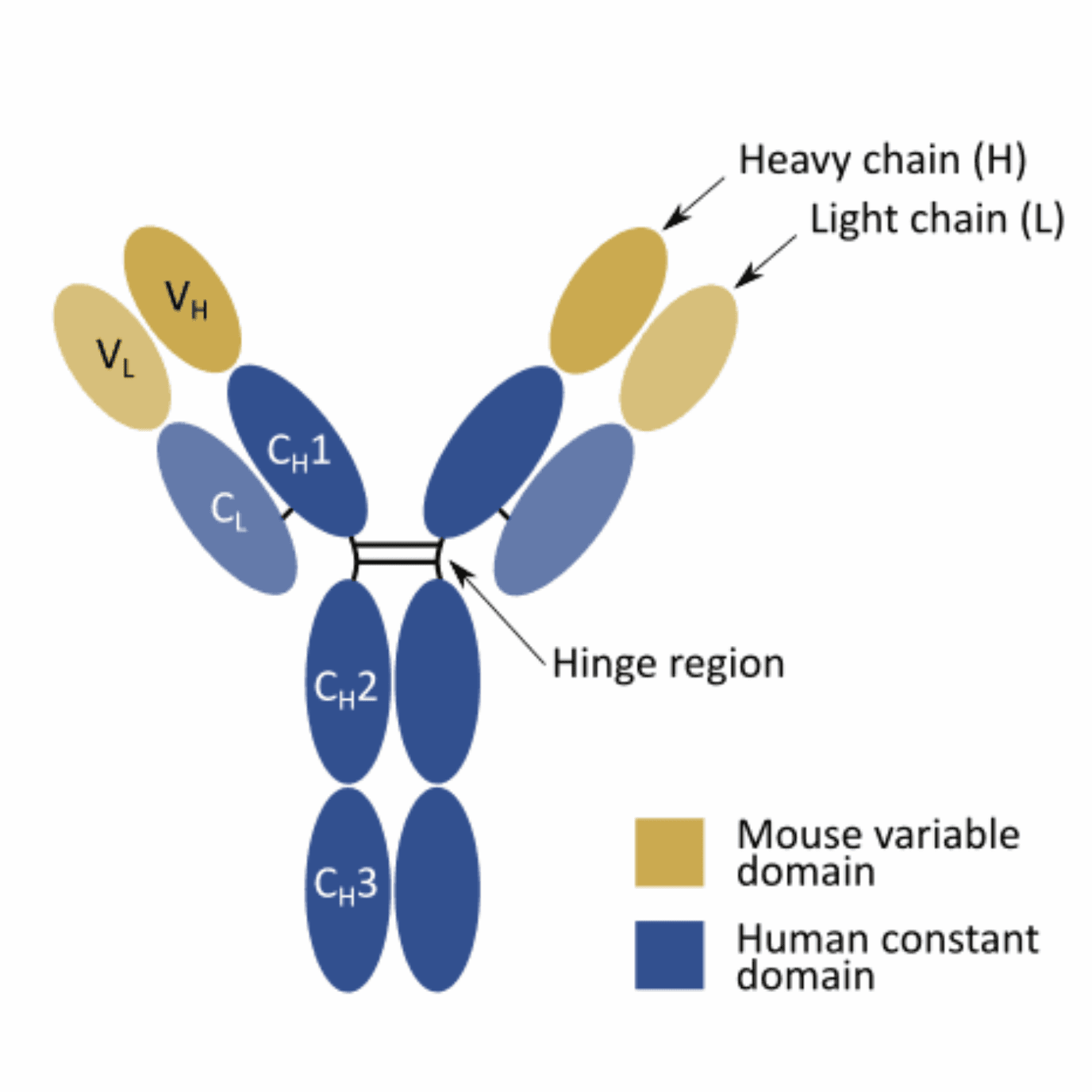
With the onset of recombinant technology, the production of these structural chimeras became very straightforward. Typically, they can be generated by first obtaining multiple hybridoma cell lines expressing a xenogeneic antibody against a specific target. The mRNA is subsequently isolated from these cell lines, and the variable domains amplified and sequenced.
Once we determine the sequence of the variable domains, we can combine them with the constant domains of an antibody from a different species and express the chimera recombinantly in a suitable expression system (e.g. mammalian, microbial, plant, or insect cell lines).
Applications of chimeric antibodies
In 1997, the anti-CD20 chimeric antibody rituximab (MabThera) became the first approved by the FDA for the treatment of cancer. Rituximab went to become one of the most successful anti-cancer biotherapeutics around the world and has been recurrently used to treat a wide range of disease including different blood cancers and autoimmune diseases.
But as of March 2020, only 8 other chimeric antibodies have been granted approval by the FDA or EMA, representing a little over 10% of all antibodies currently used in the clinic.
The latest chimeric antibody to be granted approval was isatuximab (Sarclisa®), designed to target CD38 (ADP-ribosyl cyclase 1). The chimeric mouse/human IgG1 molecule, developed by ImmunoGen and Sanofi-Aventis, is now being used to treat patients with relapsed refractory multiple myeloma. Moreover, this antibody may prove to be effective in the treatment of hematologic malignancies.
The reduced rate of approvals of chimeric antibodies in contrast with its humanized and human counterparts reflects declining interest in developing chimeric antibodies for clinical applications. However, in many cases, chimeric molecules are still widely used in the initial stage of antibody humanization strategies. And since humanized antibodies still represent most antibodies (47%) granted clinical approval, the production of chimeric intermediates is still very important.
Beyond the clinic, chimeric antibodies have proven to be useful tools for immunoassay development such as ELISA. Since these chimeric constructs can be easily produced using recombinant technologies, they are often preferred to serum-based calibrators or controls for immunoassays. They have also proven to be useful for clinical diagnostics.
For instance, serological immunoassays aiming to detect specific antibodies in patient’s samples typically rely on seropositive plasma for calibrators or controls. However, serum suffers from the same drawbacks as polyclonal antibodies:
- batch-to-batch variability
- difficulties in scaling-up production or obtaining high titers
- limitations in terms of characterization
The use of recombinantly produced chimeric antibodies allows for scalability and standardization of these important controls and calibrators. Moreover, studies show they have comparable reactivity to human serum. For this reason, these chimeras represent an important compromise that allows researchers to obtain active and standardize antibodies for their assays, without the need to fully humanize them, which would exacerbate the cost and developmental time-frame of these reagents.
Concluding remarks
Chimeric antibodies, once created to minimize the HAMA response, are now only sparsely developed for clinical applications. Today, they represent about 10% of all antibodies ever granted approval for clinical applications and are mostly used as intermediate molecules in antibody humanization.
Despite the decline in the use of this specific antibody format, it is possible these antibodies will continue to be useful as controls and calibrators in immunoassays developed to respond to specific research needs or diagnostics applications.
- Chintalacharuvu, K. R. and Morrison, S. L. Chimeric Antibodies: Production and Applications. Methods. 1995; 8(2):73-82. doi: 10.1006/meth.1995.9999
- Hackett, J. Jr et al. Recombinant mouse-human chimeric antibodies as calibrators in immunoassays that measure antibodies to Toxoplasma gondii. J Clin Microbiol. 1998; 36(5):1277-1284. Available from https://www.ncbi.nlm.nih.gov/pubmed/9574691
- Jones, M. L. and Barnard, R. T. Use of Chimeric Antibodies as Positive Controls in an Enzyme-Linked Immunosorbent Assay for Diagnosis of Scrub Typhus (Infection by Orientia tsutsugamushi). Clin Vaccine Immunol. 2007; 14(10): 1307–1310. doi: 10.1128/CVI.00114-07





