 Antibody production
Antibody production
Primary antibodies and ELISA – all you need to know to design your immunoassay
Immunoassays use primary antibodies for the detection and quantification of specific antigens in different types of samples. To date, ELISA (enzyme-linked immunosorbent assay) remains one of the most popular types of immunoassays used for a multitude of applications.
What to consider when choosing the primary antibody for your custom immunoassay
Due to their versatility, scalability, and possibility of standardization, many different types of ELISA kits have been developed and are commercially available. However, most of these assays fail to meet the needs of researchers looking to detect uncommon target antigens or to increase the sensitivity and specificity of standard assays. For these reasons, the design of custom ELISA kits is often necessary for research and diagnostics.
Custom design of ELISA assays proceeds through several stages to ensure that the best choices are made regarding the choice of format and reagents, in order to meet the goals of each unique project. These decisions should be made according to the nature and relative abundance of the target antigen, and the nature of the sample.
Principles of ELISA
The concept of ELISA comprises the use of an antibody-enzyme conjugate as a marker to detect the presence of a specific target antigen in a given sample. This concept was published independently and simultaneously by three different groups in 1971: Eva Engvall and Peter Perlman (Sweden); Stratis Avrameas and Brigitte Guilbert (France); and Anton Schuurs and Bauke van Weemen (The Netherlands).
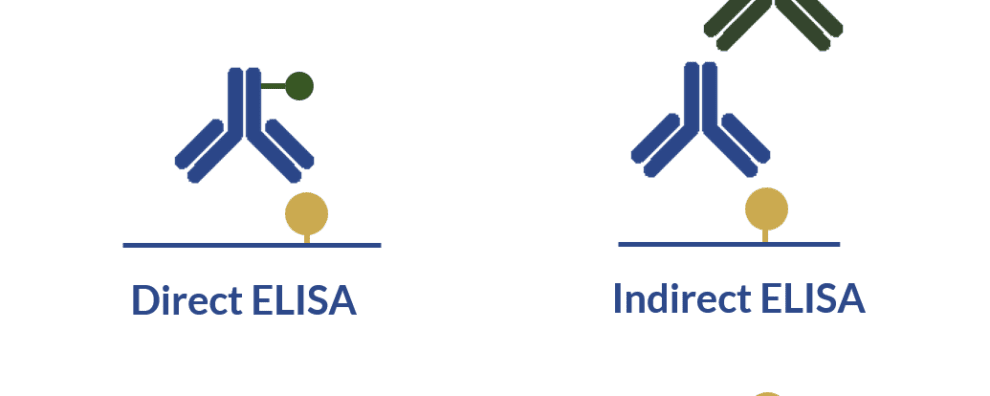
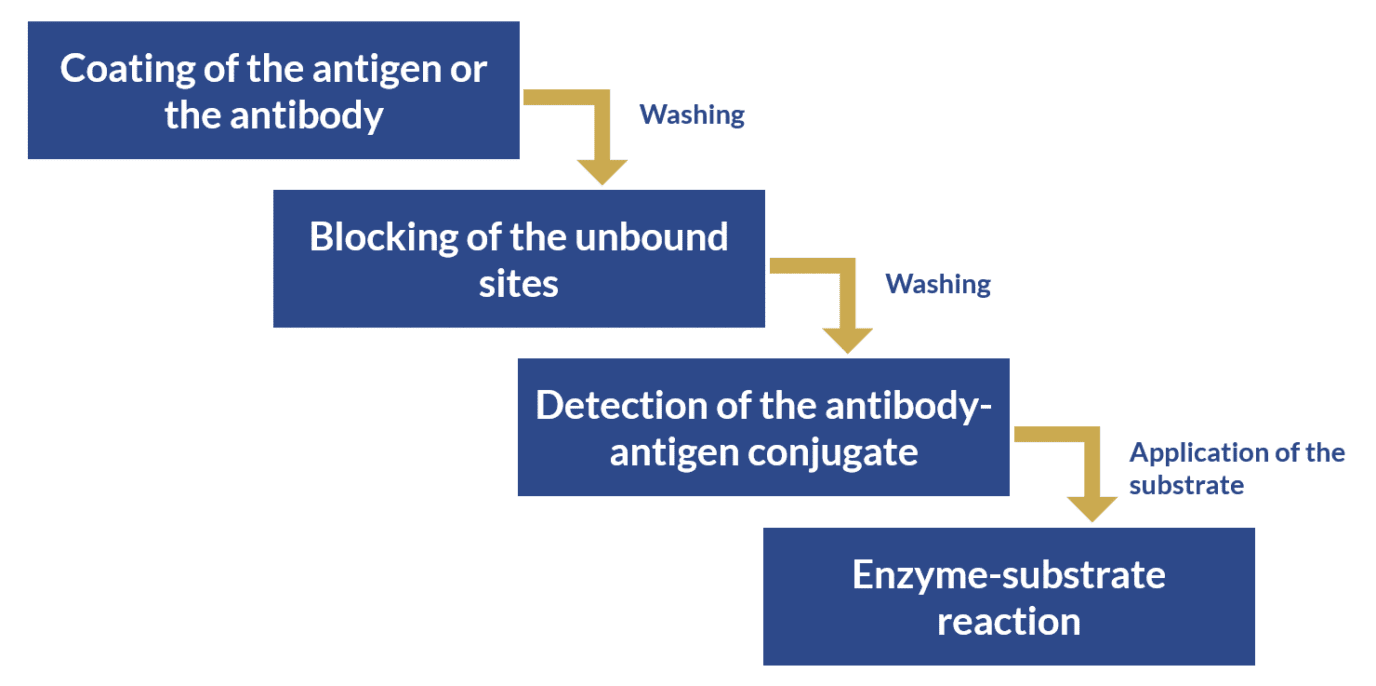
In this assay, the antibody or the antigen is bound to a solid phase (usually a microtiter plate), which allows the separation of unbound molecules by simple washes instead of time and labor-intensive separation methods.
These assays can also be coupled with a chromogenic substrate, which are colorless but release colored products when transformed by specific enzymes. For this reason, the amount of the target antigen can be quantified by measuring substrate conversion in the form of color intensity (optical density) on a spectrophotometer.
The general process for the detection or quantification of a target antigen by ELISA follows is depicted below:
What types of labels and antibodies are used in ELISA?
Antibodies used in ELISA can be classified according to the types of molecules they target. Primary antibodies are immunoglobulins designed to target the antigen of interest (protein, peptide, DNA, among others). While secondary antibodies are immunoglobulins designed to target the primary antibody.
For this reason, secondary antibodies can be engineered to recognize the constant domain (Fc) of the primary antibodies. The Fc region is shared by many different types of primary antibodies, and because of this, the use of secondary antibodies makes an assay extremely versatile since the same secondary antibody can be coupled with antibodies that target different antigens.
Secondary antibodies can be monoclonal or polyclonal, depending on if the assay requires a higher specificity or a higher sensitivity, respectively. However, due to their increased sensitivity and stability, the use of polyclonal as the secondary antibody is the most popular format used in the development of ELISA assays.
Whereas primary antibodies are preferably monoclonal to guarantee a higher degree of specificity. However, in some cases, the use of primary polyclonal antibodies was shown to increase the sensitivity and specificity of ELISA assays targeting the human growth hormone or osteolysis biomarkers.
ELISA assays using only primary antibodies, are considered simpler and less time-consuming. There’s also a reduced risk of cross-reactivity. However, because the antibody will only recognize a single antigen, these assays are also, in turn, less flexible and they have reduced signal amplification, making them less ideal to detect low-abundance antigens.
In turn, immunoassays relying on secondary antibodies are more complex, labor-intensive, time-consuming and they also have the added risk of false positives due to cross-reactivity issues. However, this indirect labeling of the target does possess some advantages.
For instance, since secondary antibodies can bind to many different primary antibodies, this type of assays becomes more flexible and allows signal amplification, which, in turn, results in greater sensitivity and helps in the detection of low-abundance targets.
Several precautions can be taken to decrease the risk of cross-reactivity in assays using both primary and secondary antibodies. For instance, if these antibodies are developed in different hosts (e.g. primary antibody in mouse and secondary antibody in rabbit) there’s a lower risk of the secondary antibody binding to itself and generating false-positive signals.
Due to the diversity and availability of many different ELISA formats, today, these assays can be designed to detect and quantify many different molecules.
Advantages and limitations of different types of ELISA assays
| Description | Advantages | Disadvantages | ||
|---|---|---|---|---|
| Direct ELISA | 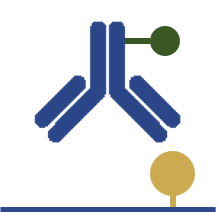 |
An antigen is coated to the wells of a microplate and its detection proceeds by direct binding of a primary antibody-enzyme conjugate.
In this assay, the amount of the target antigen is directly correlated to the signal intensity. |
Quick and simple
Highly specific No risks of cross-reactivity |
All antibodies need to be conjugated
Low flexibility since it cannot be used for multiplexing High background |
| Indirect ELISA | 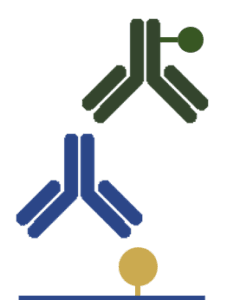 |
The detection proceeds through 4 stages:
(i) Coating of the wells with the antigen (ii) Application of an unlabeled primary antibody that binds the target antigen (iii) Wash step to remove the unbound antibody (iv) Application of an enzyme-labeled secondary antibody to bind the primary antibody In this assay, the amount of the target antigen is directly correlated to the signal intensity. |
Higher sensitive than direct ELISA due to the signal amplification
Increased flexibility due to the ability to use the same secondary antibody-enzyme conjugate to detect different primary antibodies Cost-effective Many secondary antibody-enzyme conjugates are already available |
Longer and more complex protocol
Increased risk of cross-reactivity and unspecific binding |
| Immunometric/ Sandwich ELISA | 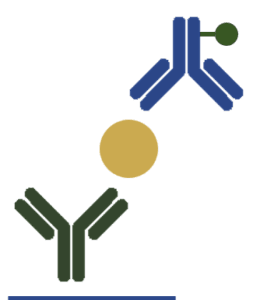 |
This format uses matched antibody pairs that bind to different and non-overlapping epitopes of the same antigen.
The detection of the antigen also proceeds through 4 steps: (i) Coating of the wells with the first antibody (capture antibody) (ii) Application of the sample (iii) Wash to remove the unbound antigen (iv) Application of the second antibody (detection antibody) to bind the immobilized antigen In this assay, the amount of the target antigen is directly correlated to the signal intensity. |
Highly specific due to the use of two antibodies for capture and detection
More sensitive than direct and indirect ELISA Suitable for complex samples since it does not require antigen purification It can also be coupled with indirect detection methods to increase its flexibility |
The development is costly and time-consuming since it can be hard to find two antibodies that bind to different regions of the same antigen
It can only be used for the detection of large antigens since the two antibodies can bind to non-overlapping epitopes Increased risk of cross-reactivity between the capture and detection antibodies |
| Competitive/ Inhibition ELISA | 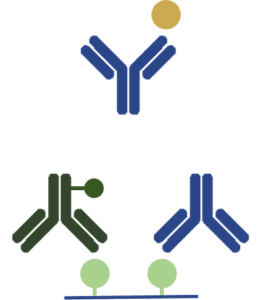 |
The competitive assay is the most complex format of ELISA.
It requires the use of an inhibitor antigen. (i) Coating of the wells with the inhibitor antigen which can bind to the primary antibody (ii) Incubation of the primary antibody with the target antigen (iii) Application of the sample containing free primary antibodies and antigen-antibody complexes to the coated microplate. Only the free antibodies will bind to the inhibitor antigen (iv) Wash to remove the unbound antibody-antigen complex (v) Application of the secondary antibody-enzyme conjugate that reacts with the antibody-inhibitor antigen complex In this assay, the amount of the target antigen is inversely correlated to the signal intensity. |
All the previous assays can be adapted to the competitive format
More robust than sandwich ELISA due to being less sensitive to sample matrix and sample dilution Ideal to detect smaller molecules that can only be detected by a single antibody Increased consistency between technical replicates It can also be coupled with indirect detection methods to increase its flexibility |
The development of these assays is costly and complex
Requires the synthesis and purification of an inhibitor antigen |
Concluding remarks
Custom ELISA assays are powerful and versatile tools used in research and diagnostics to detect and quantify different antigens across many different types of clinical and environmental samples.
Currently, both sandwich and competitive ELISA formats offer the most robust approaches to measure antigens without prior sample purification. Nevertheless, these formats can be combined and adapted to the different needs of each unique project.
- Avrameas S. and Guilbert B. A method for quantitative determination of cellular immunoglobulins by enzyme-labeled antibodies. Eur J Immunol. 1971;1(5):394-396. doi: 10.1002/eji.1830010518
- Engvall E. and Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971;8:871–874. doi: 10.1016/0019-2791(71)90454-x
- Grimaud, E. et al. Receptor Activator of Nuclear Factor κB Ligand (RANKL)/Osteoprotegerin (OPG) Ratio Is Increased in Severe Osteolysis. Am J Pathol. 2003; 163(5):2021-2031. doi: 10.1016/s0002-9440(10)63560-2
- Moura, J. F. et al. ELISA for Determination of Human Growth Hormone: Recognition of Helix 4 Epitopes. J Biomed Biotechnol. 2004; 2004(3): 143–149. doi: 10.1155/s1110724304308090
- Van Weemen B. K. and Schuurs A. H. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971;24;15(3):232-236. doi: 10.1016/0014-5793(71)80319-8





