 Antibody production
Antibody production
Fully human monoclonal antibody discovery: technologies and trends
The proportion of fully human monoclonal antibodies gaining clinical approval has quickly escalated since the approval of Humira® in 2002. Since then, 30 monoclonal antibodies and 1 bispecific antibody fragment gained approval to treat a wide range of diseases. As the demand for these biopharmaceuticals increases, so does the need to increase the efficiency of current fully human monoclonal antibody discovery technologies. What are the advantages and limitations of current approaches? How will these approaches evolve in the next decades?
Early approaches to fully human monoclonal antibody discovery
The first antibodies were generated in mice. Prompted by the revolutionary development of hybridoma technology, researchers soon discovered a way to produce sufficient amounts of these biomolecules to test their therapeutic effectiveness.
However, with the discovery of the therapeutic potential of these antibodies, came the realization that molecules from xenogeneic sources caused immunogenic reactions in patients. These reactions became known as the human anti-mouse antibody (HAMA) response.
The discovery of the HAMA response propelled the further development of methods to decrease the immunogenicity of xenogeneic antibodies (i.e. humanization). Moreover, fast-paced technological progress has since allowed the generation of fully human monoclonal antibodies.
Today, these fully human molecules are recurrently obtained by phage display and hybridoma development from transgenic mice. Since the approval of the first fully human monoclonal antibody – Humira® in 2002 through phage display of human antibody libraries, the number of fully human monoclonal antibodies approved for therapy has continued to rise, year after year.
As of December 2019, 30 fully human monoclonal antibodies and 1 fully human bispecific antibody (Lumoxiti®) have been approved for clinical use. From the 30 mAbs, 8 were generated by screening complex human antibody libraries using phage display technologies, while the remaining 22 mAbs resulted from development in transgenic mice.
Advantages and limitations of fully human monoclonal antibody generation technologies
Although most fully human monoclonal antibodies are generated in transgenic mice, established approaches to this methodology still present several limitations. For instance, although transgenics can generate highly specific and affinity-matured antibodies, they still suffer from constraints regarding antigenicity and the natural immune response of the host. For this reason, toxic antigens cannot be used to immunize mice and highly valuable antigen/targets may not elicit an adequate immune response in these hosts.
On the contrary, phage display technologies have great potential for scalability, parallelization, and miniaturization. However, these approaches don’t preserve the natural pairing information between light and heavy chain, often resulting in unnatural antibody pairing and leading to reduced affinity. Moreover, most antibodies generated by phage display may need to undergo further in vitro affinity maturation processes, that are naturally accomplished in immunized transgenic mice.
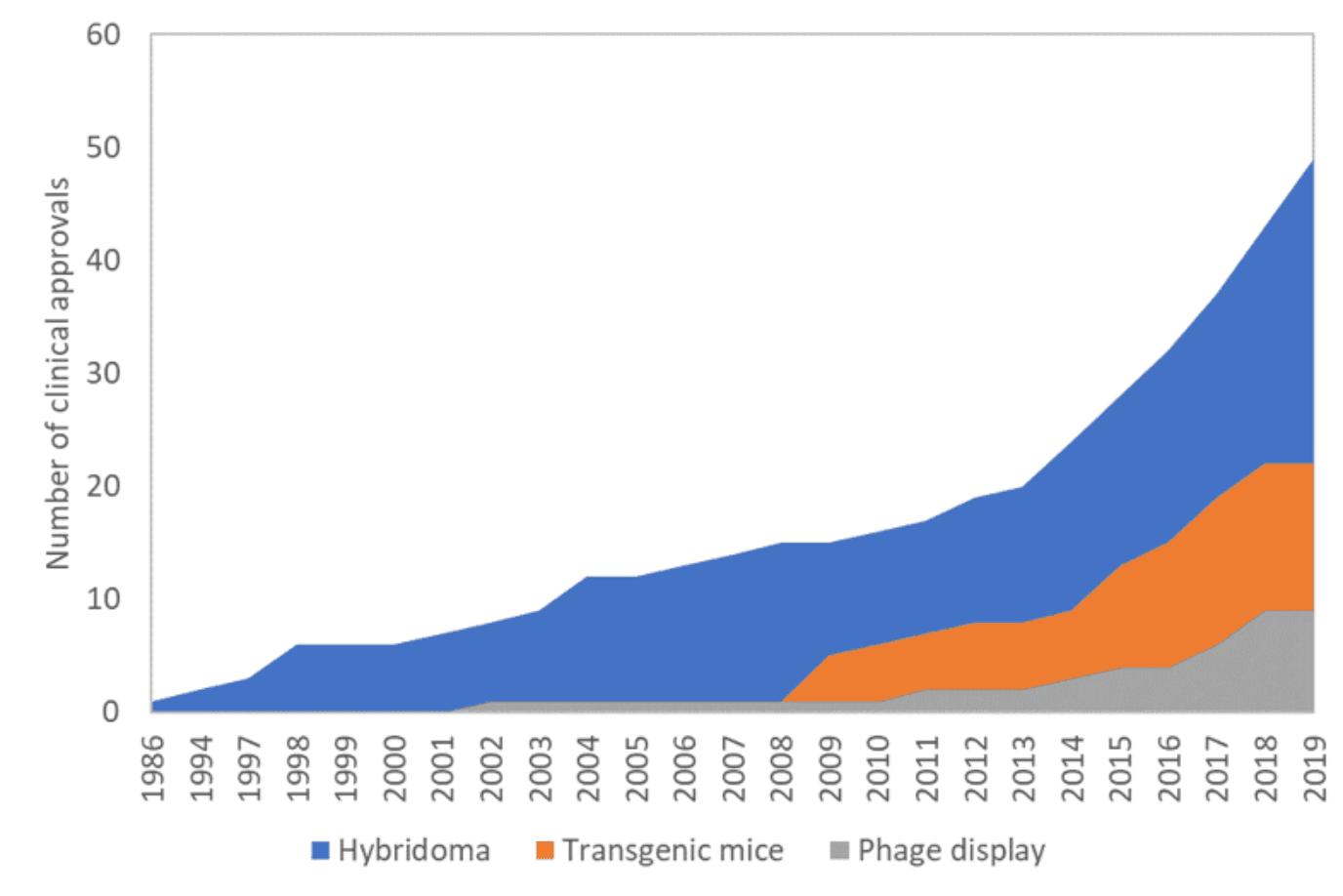
The proportion of therapeutic antibodies generated in transgenics has been steadily increasing. Experts disagree on the reasons that might explain this trend, as both transgenics-derived and phage display-derived antibodies have comparable clinical efficiency.
Independently from the reason behind the popularity of each approach, both technologies still have considerable room for improvement. For instance, transgenics will benefit from the impending transition from the low-yield hybridoma approach to the high-throughput and high-yield B cell cloning methodology.
Moreover, antibody display will likely focus on yeast and mammalian display systems to overcome the difficulties in expressing antibodies in bacterial systems. Moreover, the technology should also become more efficient by coupling the traditional display systems with FACS sorting analysis and next-generation sequencing.
Concluding remarks
It is undeniable that there has been an increasing demand for fully human monoclonal antibodies for therapeutic applications. In recent years, two technologies have predominated the discovery of these highly-sought biopharmaceuticals: phage display and antibody generation in transgenic mice.
Although the latter has garnered increasing popularity over time, both technologies are expected to play a crucial role in the future of fully human monoclonal antibody discovery. Moreover, with the advent of single-cell technologies and next-generation sequencing, both approaches are expected to evolve rapidly to overcome their current limitations.
- Lonberg, N. Fully human antibodies from transgenic mouse and phage display platforms. Curr Opin Immunol. 2008; 20(4):450-459. doi: 10.1016/j.coi.2008.06.004
- Strohl, W. E. Mouse vs Pan. The Antibody Society. July 25, 2017. Available from https://www.antibodysociety.org/mouse-vs-pan/




