 Antibody production
Antibody production
How camelid heavy-chain antibodies intensified the development of antibody engineering techniques
The discovery of functional antibody fragments and heavy-chain antibodies has intensified and propelled the development of innovative antibody engineering techniques. Through the use of these techniques, researchers have continued to challenge the canonical structure of antibodies to make them more stable and facilitating their diffusion across tissues. These efforts are paving the way for the development of more effective immunotherapies.
Antibody engineering and the discovery of functional antibody fragments
For the past decades, many therapeutic antibodies from different origins and formats have been developed and approved for the treatment of malignant, infectious and auto-immune diseases. The success of these molecules resides in their ability to bind to other biomolecules and interact with the patient’s immune system through Fc cell receptors on the surface of macrophages and natural killer cells or by activating the complement cascade.
To this date, more than 80 therapeutic antibodies have been approved for clinical use around the world. Many of them are IgG-like monoclonal antibodies (mAbs) generated in different hosts.
These numbers make mAbs one of the most important class of biopharmaceutical tools for modern medicine. But despite the great advances achieved by therapeutic mAbs, scientists continue to devise new antibody engineering techniques to overcome their inherent limitation: poor tissue diffusion.
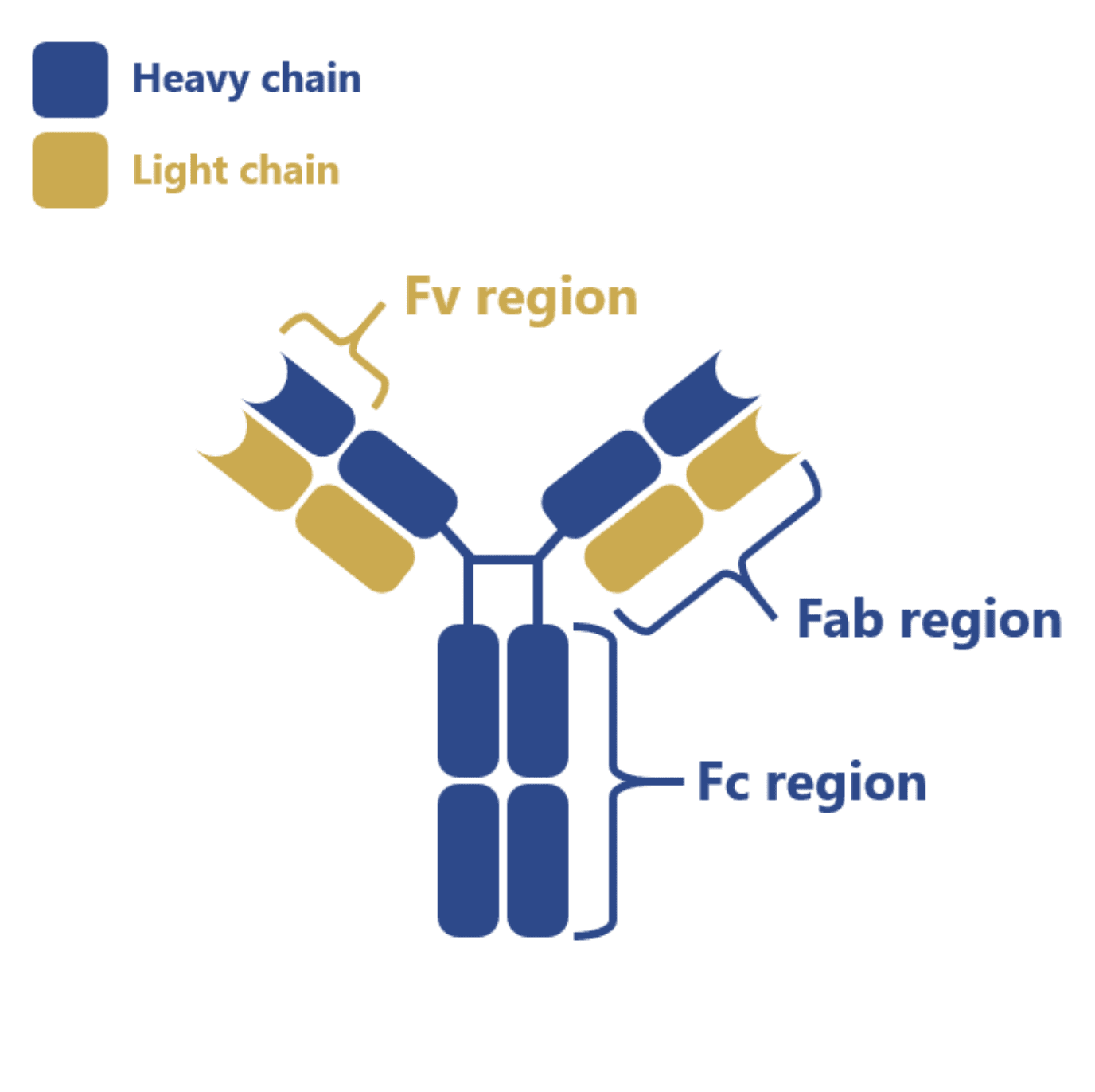
Antibodies consist of a bivalent globular structure with two variable regions (Fab), with a light (VL) and heavy (VH) domain, and a single conserved region (Fc) with two VH domains. Averaging 150 kDa, mAbs suffer from weak tissue penetration and poor pharmacokinetics and thus, it is estimated that only 20 % of administered mAbs reach their target and take effect.
These intrinsic limitations have prompted the development of multiple antibody engineering techniques that continue to push back the boundaries of possibility for immunotherapeutic applications. For the past decades, researchers have continuously challenged the canonical structure of these molecules by studying the functionality and application of antibody fragments.
The use and study of antibody fragments was further enhanced by the development of next-generation sequencing technologies and in vitro antibody display technologies, such as phage display. These technologies allowed researchers to screen many different types of antibody fragments against different antigens (binders) at large-scale.
Antibody generation and production using the phage display technology can be achieved by the following steps:
- Construction of the antibody library (naïve or immune)
- Expression of functional antibody fragments on the surface of the phage
- Displaying of the fragments, and subsequent testing of their affinity towards specific binders
- Washing of low-affinity fragments
- Elution and amplification of high-affinity fragments
- Repetition of panning cycles (3) to (5) to further enrich the most high-performing fragments
- Engineering of fragments to tailor their properties for specific applications (e.g. humanization, affinity maturation through mutation of the antibody sequences, etc.)
- Production of fragments in high-performing microbial systems (e.g. Escherichia coli, Saccharomyces cerevisiae, Pichia pastoris, etc.)
These techniques allowed the development of fully functional single-chain antibody fragments (scFv), resulting from the fusion of the VL and the VH domains of the Fv region of mAbs, among other conjugated molecules.
Furthermore, they made it simpler to isolate molecules with specific physicochemical properties. For instance, panning antibody fragments in the presence of proteases increases their subsequent resistance towards these enzymes.
However, these fragments still have limited application for the treatment of central nervous system (CNS) diseases or imaging of hard-to-reach tissues. Although they have many advantages in comparison to the use of full-length IgG-like monoclonal antibodies, they still suffer from limited solubility, tendency to form aggregates and limited diffusion across tissues, blood-brain (BB) and blood-cerebrospinal fluid (CSF) barriers.
The discovery of even smaller functional antibody fragments
All this started to change when researchers pushed these boundaries even further and discovered the potential of single-domain antibodies.
The proof of concept was published as early as 1989 by researchers from the MRC Laboratory of Molecular Biology (Cambridge, UK). This group was able to produce, for the first time, functional single-domain antibodies (sdAbs) in Escherichia coli. Unlike scFv, which consists of the fusion of the VH and VL chains of the Fv region of monoclonal antibodies, these small molecules consisted solely of a single VH domain.
Moreover, contrary to scFv, which contain six functional complementary-determining regions (CDR) free to interact with antigens, sdAbs have only three CDR. Despite this striking difference in the amount of CDR, researchers found these antibodies had a comparable antigen-binding activity to full-length and scFv fragments.
Furthermore, these fragments were incredibly small (12-15 kDa) in comparison to scFv (30 kDa) and mAbs (150 kDa). This noticeable size reduction resulted in an increased diffusion across tissues. Moreover, it also resulted in the simplification of their recombinant expression, which led to a reduction of production costs.
However, they were still found to be prone to aggregation.
This resulted from the natural amino acid composition of VH domains originating from mAbs. The VH domain of mAbs possesses hydrophobic residues meant to interact and bind the VL domain. Unfortunately, these hydrophobic residues make the single VH fragments unstable as they are prone to interact with each other sdAbs or with other hydrophobic surfaces, considerably increasing the risk of aggregation.
Discovery of heavy-chain antibodies
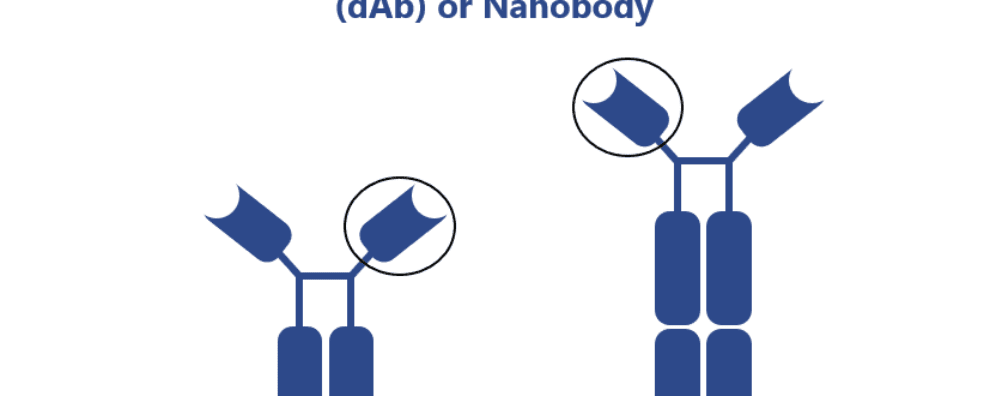
In the early 1990s, a group of researchers has further challenged our knowledge of the diversity of antibodies. They did this by describing the first naturally occurring antibodies devoid of VL domains. These antibodies, named heavy-chain antibodies (HCAbs), first described in camelids and later found in sharks, were found to have a simplified variable structure consisting of a single VH domain with three CDR.
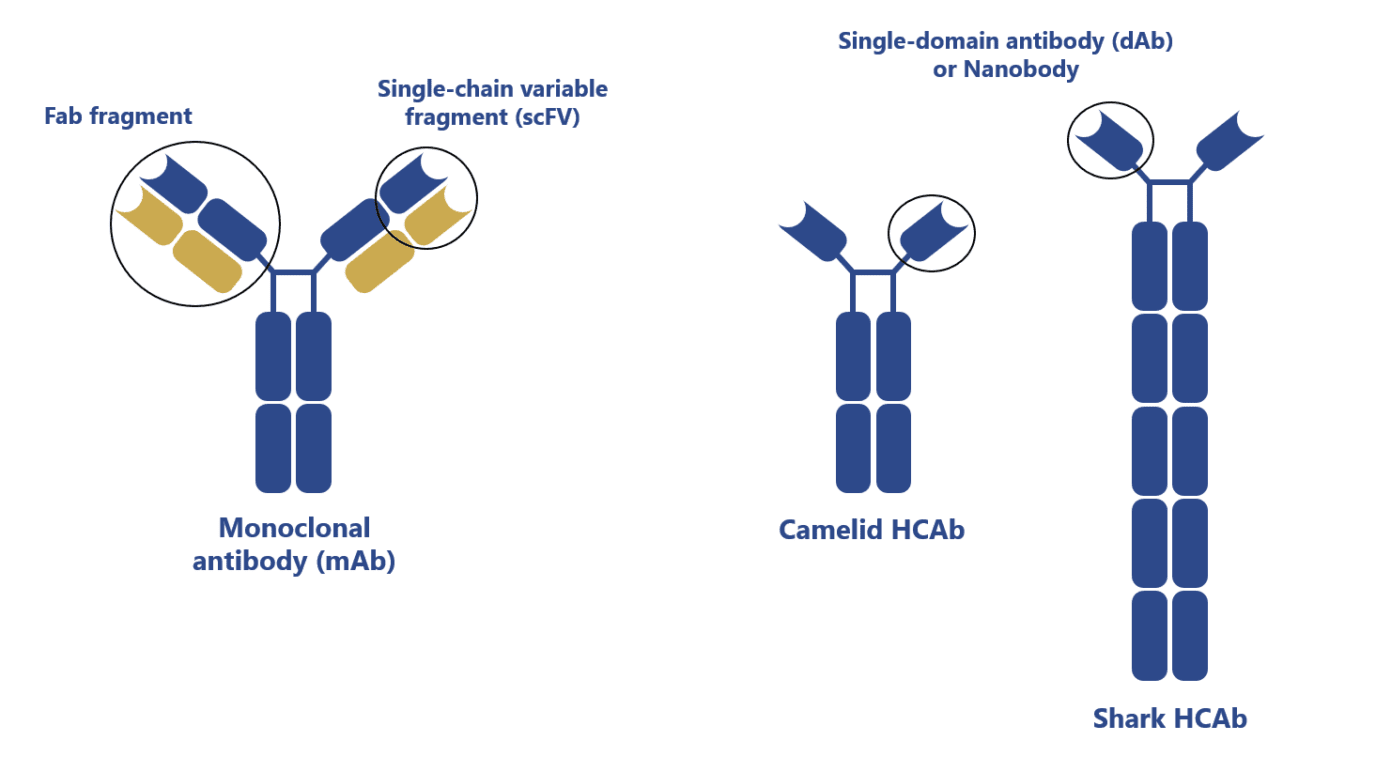
The variable regions of these antibodies were subsequently named VHHs or nanobodies. Interestingly, due to the lack of a VL domain, VHHs are fully hydrophilic, resulting in an increased solubility and lower susceptibility to form aggregates in comparison to mAbs and mAb-derived fragments.
Despite the presence of only three CDR, these fragments were shown to have high-affinity towards antigens. This strong ability is often attributed to the presence of a very long third CDR (CDR3) in VHHs in comparison to its homologous region in human mAbs. They also exhibit a high homology with human type 3 VH domains resulting in a low immunogenic burden and making them highly desirable for therapeutic applications.
Moreover, the structural simplicity and ease of production of VHH makes them ideal frameworks for antibody engineering approaches. Thus, many engineered versions of HCAbs are currently being developed for different therapeutic applications:
- Full-length HCAbs
- Chimeric camelid-human antibodies, with camelid VHH and human Fc regions
- Humanized HCAb, produced by graphing human sequences into the VHH domain of camelid antibodies
- “Camelized” HCAbs, fully heavy chained human antibodies modified by the introduction of hydrophilic residues in the hydrophobic interface of human VH
- Bispecific HCAbs
- Full-length bispecific HCAbs, consisting of the fusion of additional VHH in the framework of a natural HCAb
- BiTE Nanobodies (Bispecific T-cell engager), two VHH with different affinities genetically linked to each other
- Multimeric nanobodies, two or more similar VHH domains fused through the use of flexible glycine-serine linkers
- Nanobody conjugates
- Nanobody-toxin conjugates also called immunotoxins
- Nanobody-radionuclide
- Nanobody-fluorochrome
- Nanobody-drug
Despite being described as early as the 1990s, and despite recent advances in the understanding and engineering of nanobodies, the first therapeutic antibody based on camelid HCAbs was only recently approved.
Developed by Sanofi, this therapeutic antibody, named caplacizumab (Cablivi®, DrugBank accession number DB06081), is a bivalent VHH antibody, and the first single-domain antibody to get EMA and FDA approvals in 2018 and 2019, respectively. Since then it has been in use for the treatment of a life-threatening, autoimmune clotting disorder, named acquired thrombocytopenic purpura (aTTP).
sdAbs for CNS diagnostics and therapy
The treatment of CNS disorders is yet one of the most complex challenges of modern medicine. This happens because the BB barrier acts as a chemical gatekeeper preventing foreign molecules to cross the barrier and reach the brain.
The BB barrier consists of specialized endothelial cells forming an impenetrable physical wall of tight junctions. Furthermore, the BB barrier contains many efflux transporters responsible for eliminating unwanted molecules from the brain. This restrictive physiology is necessary to protect the brain, but it also severely limits the delivery of CNS-targeting therapeutics.
One way to circumvent this limitation is to make use of natural transport systems that shuttle important molecules (e.g. vitamins, proteins and other nutrients) between the blood and the brain.
Being smaller and more soluble than conventional mAbs, VHHs were found to have a greater potential for crossing the BB barrier through these transporters. In fact, studies with FC5 and FC44, two camelid VHHs obtained from a naïve library, showed that these fragments accumulated at high levels in the brain following tail vein injection in rodents.
This accumulation was even more pronounced when FC5 was fused to a human IgG1 Fc region. Further suggesting that VHH in monomeric form or as Fc-VHH constructs have the potential to increase the efficiency of treatment and diagnostics of CNS diseases.
Concluding remarks
The term antibody engineering involves the manipulation of the canonical structure of antibodies to increase their solubility and stability. For the past three decades, this has evolved from the manipulation of full-length antibodies to the manipulation of camelid-derived VHH.
VHHs continue to intensify the research on antibody engineering techniques to increase their therapeutic efficiency. Recently, researchers have obtained promising results in the use of VHHs to cross the BB barrier. These results have propelled a renewed interest in the use of these effective biopharmaceuticals for the treatment of CNS diseases.
- Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Retrieved from: https://www.antibodysociety.org/resources/approved-antibodies/ Accessed on: October 2019
- Arbabi-Ghahroudi, M. Camelid Single-Domain Antibodies: Historical Perspective and Future Outlook. Front Immunol. 2017; 8:1589. doi: 10.3389/fimmu.2017.01589
- Bannas, P. et al. Nanobodies and Nanobody-Based Human Heavy Chain Antibodies as Antitumor Therapeutics. Front Immunol. 2017; 8:1603. doi: 10.3389/fimmu.2017.01603
- Bazan, J. et al. Phage display—A powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum Vaccin Immunother. 2012; 8(12):1817–1828. doi:10.4161/hv.21703
- Bélanger, K. et al. Single-Domain Antibodies as Therapeutic and Imaging Agents for the Treatment of CNS Diseases. Antibodies (Basel). 2019; 8(2):27. doi: 10.3390/antib8020027
- Greenberg, A. S. et al. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995; 374(6518):168-73. doi: 10.1038/374168a0
- Hamers-Casterman, C. et al. Naturally occurring antibodies devoid of light chains. Nature. 1993; 363(6428):446-8. doi: 10.1038/363446a0
- Kunz, P. et al. Exploiting sequence and stability information for directing nanobody stability engineering. Biochim Biophys Acta Gen Subj. 2017; 1861(9):2196-2205. doi: 10.1016/j.bbagen.2017.06.014
- Stanimirovic, D. B. et al. Emerging Technologies for Delivery of Biotherapeutics and Gene Therapy Across the Blood-Brain Barrier. BioDrugs. 2018; 32(6):547-559. doi: 10.1007/s40259-018-0309-y
- Ward, E. S. et al. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989; 341(6242):544-6. doi: 10.1038/341544a0




