 Protein production
Protein production
Applications of the XtenCHO™ transient expression system
XtenCHO™ combines the high productivity of a new CHO-derived cell line with the best reagents for the rapid production of complex glycoproteins. The new system was specifically designed to support early drug discovery, protein engineering, and research applications. Read this article to learn more.
How XtenCHO™ supports research and drug discovery
XtenCHO™ is a mammalian cell-based transient host developed from a highly productive Chinese Hamster Ovary (CHO) cell line. The original host has been extensively engineered to maximize protein production and processing, resulting in superior secretion and folding, delayed apoptosis, and efficient protein glycosylation.
Together with an optimal growth medium (chemically defined and animal-free), efficient one-step transfection reagent, and high expression vector, XtenCHO™ achieves up to 10x higher yields and maintains consistent expression levels for more than 14 days, 3x longer than most commercially available systems. Given its optimal protein folding and post-translational modifications, XtenCHO™ is ideal for the production of small to medium-scale quantities of monoclonal antibodies and other complex and soluble proteins (e.g., enzymes, soluble receptors, among others).
This transient expression system was designed to cater to three major applications:
- Early drug discovery: potential drug candidate screening
- Protein engineering: variant screening
- Research: analytical reagents
Shop these products
Using XtenCHO™ in early drug discovery
Developing a new drug is a complex process, taking up to a decade or more to bring an early concept to the clinic and entailing investments in the order of the hundreds of thousands. Despite the industry’s best efforts, the majority of early candidates (>90%) never make it into clinical trials. Unexpected developability or scalability problems are often cited as the main culprits of this high failure rate.
Experts have identified several strategies to overcome current drug development constraints. The most important of these entail early screening and bioanalysis of drug candidates in transient systems. But what makes a host optimal for studying the developability profile of a new drug?
- The transient systems must be able to produce reasonable quantities of several candidate drugs in a short timeframe and with little need for extensive process optimization
- The transient host must be able to grow in chemically defined media to avoid animal component contamination and extensive protein purification processes
- The system must also be similar to the stable expression system that will be used for biopharmaceutical-grade production to minimize scalability issues
XtenCHO™ was designed to answer these needs. Endowed with smart protocols for easy integration into existing workflows, this new system can produce monoclonal antibodies that are indistinguishable from those produced in stable high-quality systems. This vital feature of the system allows the detection of developability issues early during the process (e.g., high aggregation, low stability at high temperatures, among others) and the quick transition from transient to stable biopharmaceutical-grade expression.
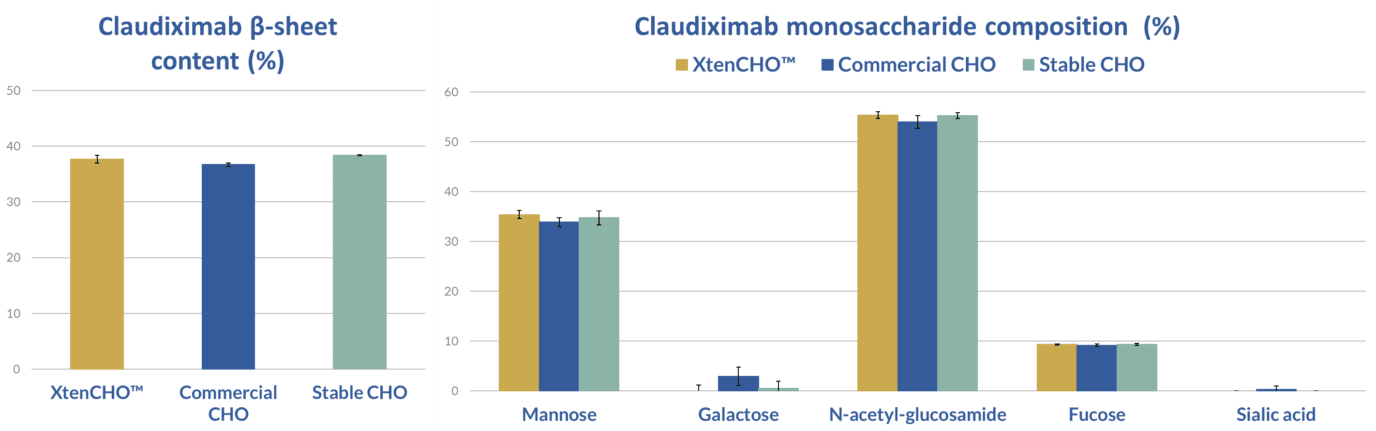
Using XtenCHO™ to boost protein engineering efforts
Often, promising proteins or mAbs require fine-tuning to fit specific applications. For instance, mAbs obtained from non-human hosts require humanization to reduce immunogenicity and increase therapeutic effectiveness. Similarly, the process of fine-tuning affinity in mAbs and enzymes is often vital to ensure these biologicals have (i) sufficient affinity towards the desired target and (ii) low affinity towards non-related but structurally similar targets.
These efforts of protein engineering span multiple fields, from therapeutic protein development to bioproduction, diagnostics, or even biosensing applications. Independently of the final application, protein engineering requires:
- Strong knowledge of a protein’s structure, domains, and function
- A robust method for generating protein variants such as in silico or site-directed mutagenesis
- A reliable and fast method for screening the variants’ key properties
Although in silico drug discovery and engineering has advanced significantly, it can only assist the process up to a certain point. Once candidates with potentially enhanced properties are identified and narrowed down to manageable numbers, protein screening becomes the vital next step. In this context, the use of a fast, reproducible, and optimized transient system can be an advantage and mitigate the risk of developability issues down the line.
At ProteoGenix, the efficiency of our protein engineering platform rests on the rapid production of protein variants in XtenCHO™ transient systems. The rapidity and reliability of the platform makes early proof-of-concept studies feasible which, in turn, maximizes the success of protein engineering processes.
Using XtenCHO™ to generate high-quality biological reagents
Monoclonal antibodies, enzymes, and other proteins are indispensable tools in our current molecular biology toolbox. Most mAbs are used in multiple immunoassays that aim at detecting, monitoring, and even quantifying proteins in vitro or in vivo. These assays are typically employed in research (medical, basic, environmental, among others) or diagnostics:
- Immunohistochemistry (IHC) or immunocytochemistry (ICC): chromogenic-labeled mAbs are used to stain specific cells (ICC) or tissues (IHC)
- Immunofluorescence (IF): similar to IHC and ICC assays but employing fluorescent labels instead of chromogenic ones
- Western Blot (WB): a method that employs mAbs to separate and detect proteins
- Immunoprecipitation (IP): a popular technique used to capture and concentrate proteins from complex mixtures
- Flow Cytometry (FC): an extremely flexible method used to detect and quantify biomarkers in liquid samples such as blood – extremely useful to study disease progression in response to specific treatments
- Enzyme-linked Immunosorbent Assay (ELISA): one of the most popular and important immunoassays, ELISA can be developed in multiple formats and allow the rapid detection and quantification of specific biomarkers in liquid samples – it is a high-throughput method and miniaturized method
- ImmunoPET: also known as immune-positron emission tomography, it combines PET with monoclonal antibodies for in vivo studies and diagnostics in real-time
Presently, mAbs used in immunoassays for research applications are primarily produced in hybridoma cell lines. These lines are stored in international or local cell banks and can be purchased or exchanged between researchers from different laboratories.
However, producing mAbs for these assays in hybridomas instead of recombinant systems comes with a downside:
- Hybridomas are prone to genetic drift, often resulting in the irreversible loss of antibody-encoding genes
- Some hybridoma cell lines grow rather poorly in chemically-defined medium and require serum to produce mAbs in reasonable amounts, leading to reagents with lower purity
- The sequence of mAbs produced in hybridomas are often unknown, making it impossible to protect them against gene loss or to improve their properties by protein engineering
These risks can be mitigated by the use of highly productive and easy-to-optimize systems like XtenCHO™. With smart protocols and reduced hands-on time, the new transient system was designed to be easily integrated into existing immunoassay workflows, making the transition from native hybridoma to recombinant production stress-free.